Standard Enthalpy Of Formation In Easy Definition . For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. The standard enthalpy change of formation of a compound is the enthalpy change which occurs when one mole of the compound is formed from its elements under standard conditions, and with. The standard enthalpy of formation of any element in its standard state is zero by definition. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the reactants and. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free.
from www.slideserve.com
193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. The standard enthalpy change of formation of a compound is the enthalpy change which occurs when one mole of the compound is formed from its elements under standard conditions, and with. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the reactants and. The standard enthalpy of formation of any element in its standard state is zero by definition.
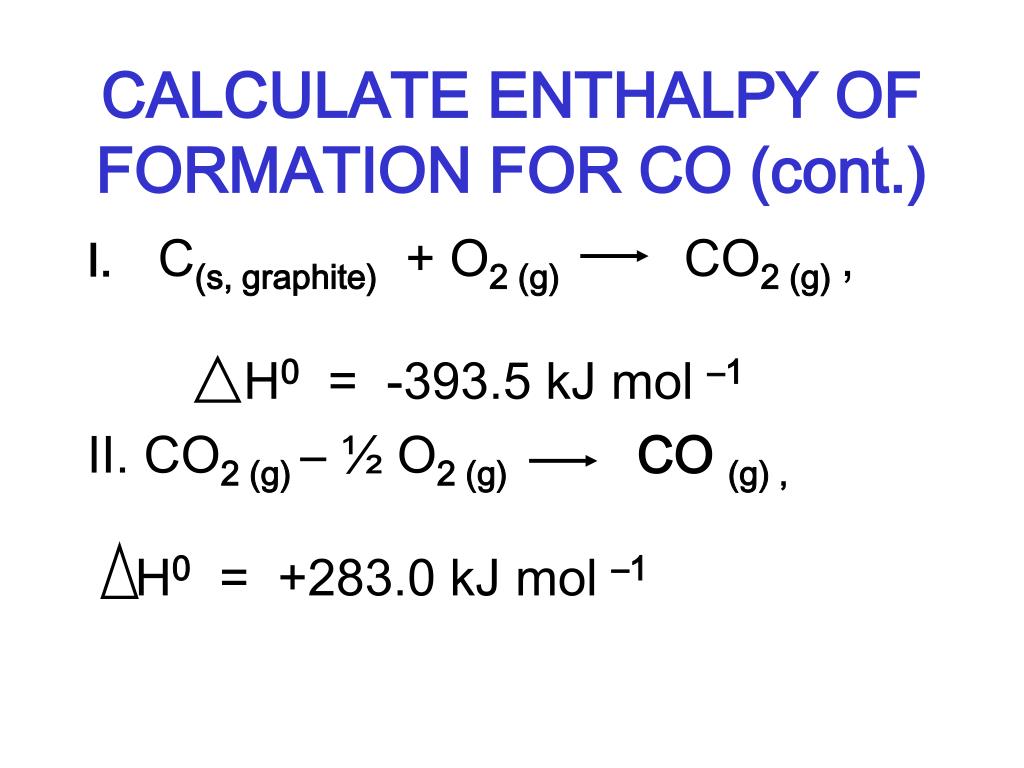
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation
Standard Enthalpy Of Formation In Easy Definition A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the reactants and. The standard enthalpy change of formation of a compound is the enthalpy change which occurs when one mole of the compound is formed from its elements under standard conditions, and with. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. The standard enthalpy of formation of any element in its standard state is zero by definition. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of.
From mungfali.com
Enthalpies Of Formation Table Standard Enthalpy Of Formation In Easy Definition For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy change of formation of a. Standard Enthalpy Of Formation In Easy Definition.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Standard Enthalpy Of Formation In Easy Definition A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy change of formation of a compound is the enthalpy change which occurs when one mole of the compound is formed from its elements under standard conditions, and with. For example, although. Standard Enthalpy Of Formation In Easy Definition.
From www.chegg.com
Solved ΔHfo equals zeroΔHc° does not equal zeroAnswer Bank Standard Enthalpy Of Formation In Easy Definition For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm. Standard Enthalpy Of Formation In Easy Definition.
From www.youtube.com
Enthalpies of Reactions Using Average Bond Enthalpies Chemistry Standard Enthalpy Of Formation In Easy Definition A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The standard enthalpy change of formation of a compound is the enthalpy change which occurs when one mole of the compound is formed from its elements under standard conditions, and with. 193 rows in chemistry and thermodynamics,. Standard Enthalpy Of Formation In Easy Definition.
From www.coursehero.com
[Solved] Using the table of standard formation enthalpies that you'll Standard Enthalpy Of Formation In Easy Definition The standard enthalpy change of formation of a compound is the enthalpy change which occurs when one mole of the compound is formed from its elements under standard conditions, and with. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. 193 rows in. Standard Enthalpy Of Formation In Easy Definition.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Of Formation In Easy Definition The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is given the symbol δfho δ f. Standard Enthalpy Of Formation In Easy Definition.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Standard Enthalpy Of Formation In Easy Definition For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm. Standard Enthalpy Of Formation In Easy Definition.
From www.youtube.com
L'enthalpie de formation YouTube Standard Enthalpy Of Formation In Easy Definition The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. The standard enthalpy change of formation of a compound is the enthalpy change which occurs when one mole of the compound is formed from its elements under standard conditions, and with. 193 rows in chemistry. Standard Enthalpy Of Formation In Easy Definition.
From www.youtube.com
Calculating Reaction Enthalpy from Enthalpies of Formation YouTube Standard Enthalpy Of Formation In Easy Definition A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The standard enthalpy change of formation of a compound is the enthalpy change which occurs when one mole of the compound is formed from its elements under standard conditions, and with. The standard enthalpy of formation of. Standard Enthalpy Of Formation In Easy Definition.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Standard Enthalpy Of Formation In Easy Definition A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the reactants and. The standard enthalpy change of formation of a compound is the enthalpy change. Standard Enthalpy Of Formation In Easy Definition.
From pdfprof.com
enthalpies standard de formation et entropie standard Standard Enthalpy Of Formation In Easy Definition A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The standard enthalpy change of formation of a compound is the enthalpy change which occurs when one mole of the compound is formed from its elements under standard conditions, and with. The standard enthalpy of formation is. Standard Enthalpy Of Formation In Easy Definition.
From www.chegg.com
Solved Score 79400Which of the following has a standard Standard Enthalpy Of Formation In Easy Definition The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy change of formation of a compound is the enthalpy. Standard Enthalpy Of Formation In Easy Definition.
From stahonorschemistry.weebly.com
III Calculating Enthalpies STA Form IV Honors Chemistry Standard Enthalpy Of Formation In Easy Definition The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the reactants and. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy change of formation of a compound is. Standard Enthalpy Of Formation In Easy Definition.
From www.chegg.com
Solved What is the standard enthalpy of formation of liquid Standard Enthalpy Of Formation In Easy Definition The standard enthalpy of formation of any element in its standard state is zero by definition. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that. Standard Enthalpy Of Formation In Easy Definition.
From www.youtube.com
Comprendre l'Enthalpie Standard de Formation YouTube Standard Enthalpy Of Formation In Easy Definition The standard enthalpy change of formation of a compound is the enthalpy change which occurs when one mole of the compound is formed from its elements under standard conditions, and with. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy. Standard Enthalpy Of Formation In Easy Definition.
From fyotefdel.blob.core.windows.net
Standard Enthalpy Of Formation Pbo at Sarah Buckner blog Standard Enthalpy Of Formation In Easy Definition The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy change of formation of a compound is the enthalpy change which occurs when one mole of the compound is formed from its elements under standard conditions, and with. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard. Standard Enthalpy Of Formation In Easy Definition.
From zeviernswenson.blogspot.com
Standard Enthalpy of Formation ZeviernSwenson Standard Enthalpy Of Formation In Easy Definition For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the reactants and. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure.. Standard Enthalpy Of Formation In Easy Definition.
From www.chegg.com
Solved Use a standard enthalpies of formation table to Standard Enthalpy Of Formation In Easy Definition The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the reactants and. For example, although oxygen can exist as ozone (o 3), atomic. Standard Enthalpy Of Formation In Easy Definition.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation Standard Enthalpy Of Formation In Easy Definition The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the reactants and. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is. Standard Enthalpy Of Formation In Easy Definition.
From darkataxa.blogspot.com
Astounding Collections Of Heat Of Formation Table Photos Darkata Standard Enthalpy Of Formation In Easy Definition 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy change of formation of a compound is the enthalpy change which occurs when one mole of the compound is formed from its elements under standard conditions, and with. The standard enthalpy of formation of. Standard Enthalpy Of Formation In Easy Definition.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Standard Enthalpy Of Formation In Easy Definition The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. For example, although oxygen can exist as ozone (o 3), atomic oxygen. Standard Enthalpy Of Formation In Easy Definition.
From rayb78.github.io
Heat Of Formation Chart Standard Enthalpy Of Formation In Easy Definition A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The standard enthalpy of formation of any element in its standard state is zero by definition. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure. Standard Enthalpy Of Formation In Easy Definition.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation In Easy Definition The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. The standard enthalpy change of formation of a compound is the enthalpy change which occurs when one mole of the compound is formed from its elements under standard conditions, and with. A standard enthalpy of. Standard Enthalpy Of Formation In Easy Definition.
From mungfali.com
Enthalpies Of Formation Chart Standard Enthalpy Of Formation In Easy Definition A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is defined as the change in. Standard Enthalpy Of Formation In Easy Definition.
From mungfali.com
Table Of Enthalpy Of Formation Standard Enthalpy Of Formation In Easy Definition The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the reactants and. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. 193 rows in chemistry and thermodynamics, the standard enthalpy of. Standard Enthalpy Of Formation In Easy Definition.
From www.youtube.com
R1.2.3 / R1.2.4 Standard enthalpy change of formation (HL) YouTube Standard Enthalpy Of Formation In Easy Definition The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the reactants and. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. A standard enthalpy of formation \ (δh^\circ_\ce. Standard Enthalpy Of Formation In Easy Definition.
From pediaa.com
What is the Difference Between Enthalpy of Formation and Enthalpy of Standard Enthalpy Of Formation In Easy Definition A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The standard enthalpy change of formation of a compound is the enthalpy change which occurs when one mole of the compound is formed from its elements under standard conditions, and with. 193 rows in chemistry and thermodynamics,. Standard Enthalpy Of Formation In Easy Definition.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Enthalpy Of Formation In Easy Definition The standard enthalpy change of formation of a compound is the enthalpy change which occurs when one mole of the compound is formed from its elements under standard conditions, and with. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. The standard enthalpy of formation of any element in its standard state is zero by definition.. Standard Enthalpy Of Formation In Easy Definition.