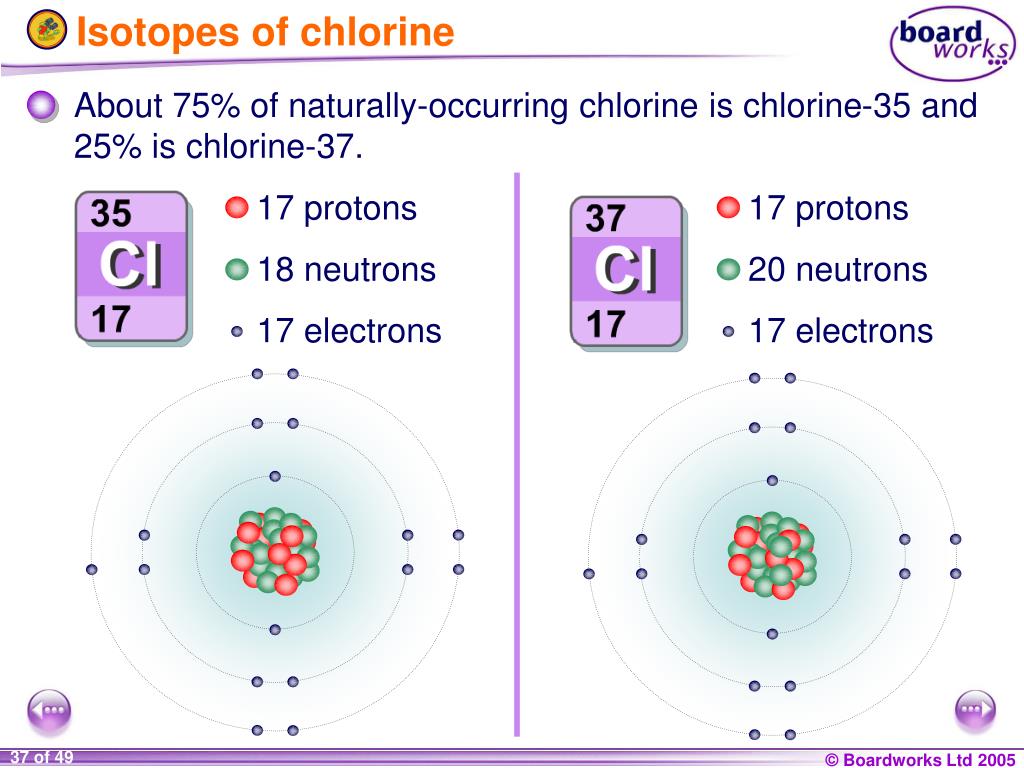
Difference Chlorine Isotopes . Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. There are two stable isotopes, 35. chlorine (cl) has isotopes with mass numbers ranging from 32 g mol −1 to 40 g mol −1. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. chlorine isotopes are variants of chlorine atoms that have the same number of protons but different numbers of neutrons, leading. the primary decay mode of isotopes lighter than 35 cl is electron capture to isotopes of sulfur; for example, the relative atomic mass of chlorine is 35.5 rather than a whole number. natural chlorine is a mixture of two stable isotopes: Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. the primary difference between chlorine 35 and chlorine 37 lies in their atomic mass. This is because chlorine contains two. There are two principal stable. This is because chlorine contains two. Unlike protons, the number of neutrons is not absolutely fixed for most elements. chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl.
from www.slideserve.com
That of isotopes heavier than 37 cl. This is because chlorine contains two. This is because chlorine contains two. There are two principal stable. these differing atoms of the same element are called isotopes. for example, the relative atomic mass of chlorine is 35.5 rather than a whole number. the primary decay mode of isotopes lighter than 35 cl is electron capture to isotopes of sulfur; 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. 13 rows chlorine ( 17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. There are only two stable.
PPT KS4 Chemistry PowerPoint Presentation, free download ID5568233
Difference Chlorine Isotopes Unlike protons, the number of neutrons is not absolutely fixed for most elements. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. this table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins,. 13 rows chlorine ( 17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. There are two principal stable. There are two stable isotopes, 35. This is because chlorine contains two. isotopes are atoms of the same element that differ in the amount of neutrons and atomic mass. the primary decay mode of isotopes lighter than 35 cl is electron capture to isotopes of sulfur; these differing atoms of the same element are called isotopes. There are only two stable. (a) when a sample of elemental. these differing atoms are called isotopes. New york city’s tap water — a source of pride for residents — might taste a bit. This is because chlorine contains two. simultaneous detection of uranium isotopes and fluorine advances nuclear nonproliferation monitoring.
From byjus.com
cl 35 and cl 37 are two isotopes of chlorine . if average atomic mass Difference Chlorine Isotopes New york city’s tap water — a source of pride for residents — might taste a bit. Four isotopes of helium (he) are shown in. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. isotopes are elements with different numbers of neutrons in the. Difference Chlorine Isotopes.
From www.numerade.com
There are two main isotopes of chlorine. 75 of chlorine atoms have a Difference Chlorine Isotopes New york city’s tap water — a source of pride for residents — might taste a bit. There are only two stable. isotopes are elements with different numbers of neutrons in the nucleus but the same number of protons. There are only two stable. for example, the relative atomic mass of chlorine is 35.5 rather than a whole. Difference Chlorine Isotopes.
From pediaa.com
Difference Between Chloroform and Chlorine Definition, Properties Difference Chlorine Isotopes for example, the relative atomic mass of chlorine is 35.5 rather than a whole number. This is because chlorine contains two. Four isotopes of helium (he) are shown in. these differing atoms of the same element are called isotopes. Unlike protons, the number of neutrons is not absolutely fixed for most elements. 13 rows chlorine ( 17. Difference Chlorine Isotopes.
From askanydifference.com
Chlorine vs Chloride Difference and Comparison Difference Chlorine Isotopes There are two stable isotopes, 35. the primary decay mode of isotopes lighter than 35 cl is electron capture to isotopes of sulfur; (a) when a sample of elemental. This is because chlorine contains two. Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. isotopes \(\ce{^35}cl\) and \(\ce{^37}cl\) are the two natural, stable. Difference Chlorine Isotopes.
From fineartamerica.com
Isotopes Of Chlorine Photograph by Photo Libary Difference Chlorine Isotopes chlorine consists of two isotopes, 35 cl and 37 cl, in approximately a 3:1 ratio. New york city’s tap water — a source of pride for residents — might taste a bit. isotopes \(\ce{^35}cl\) and \(\ce{^37}cl\) are the two natural, stable isotopes of chlorine. chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl,. Difference Chlorine Isotopes.
From www.numerade.com
SOLVEDChlorine has two natural isotopes 17^37 Cl and 17^35 Cl Difference Chlorine Isotopes isotopes are elements with different numbers of neutrons in the nucleus but the same number of protons. All atoms of chlorine (cl) have 17 protons, but there are chlorine. Isotopes are forms of an element that have the same number of protons but different. chlorine isotopes are variants of chlorine atoms that have the same number of protons. Difference Chlorine Isotopes.
From www.researchgate.net
Stable isotopes of chlorine Download Scientific Diagram Difference Chlorine Isotopes That of isotopes heavier than 37 cl. chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. these differing atoms are called isotopes. for example, the relative atomic mass of chlorine is 35.5 rather than a whole number. isotopes are elements with different numbers of. Difference Chlorine Isotopes.
From dokumen.tips
(PDF) CompoundSpecific Chlorine Isotope Analysis A Comparison of Gas Difference Chlorine Isotopes There are two stable isotopes, 35. chlorine consists of two isotopes, 35 cl and 37 cl, in approximately a 3:1 ratio. There are two principal stable. isotopes are elements with different numbers of neutrons in the nucleus but the same number of protons. Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. This. Difference Chlorine Isotopes.
From exowpfdng.blob.core.windows.net
Chlorine In Water How Long Does It Last at Richard Harris blog Difference Chlorine Isotopes isotopes are atoms of the same element that differ in the amount of neutrons and atomic mass. Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. There are only two stable. Unlike protons, the number of neutrons is not absolutely fixed for most elements. the different types of chlorine are called. chlorine. Difference Chlorine Isotopes.
From www.vrogue.co
Isotopes And Isobars Definition Uses And Difference T vrogue.co Difference Chlorine Isotopes isotopes are elements with different numbers of neutrons in the nucleus but the same number of protons. this table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins,. Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. chlorine consists of two isotopes, 35 cl and 37. Difference Chlorine Isotopes.
From www.breakingatom.com
Isotopes of Elements Difference Chlorine Isotopes for example, the relative atomic mass of chlorine is 35.5 rather than a whole number. All atoms of chlorine (cl) have 17 protons, but there are chlorine. There are two stable isotopes, 35. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. chlorine. Difference Chlorine Isotopes.
From valenceelectrons.com
Protons, Neutrons, Electrons for Chlorine (Cl, Cl) Difference Chlorine Isotopes Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. for example, the relative atomic mass of chlorine is 35.5 rather than a whole number. Unlike protons, the number of neutrons is not absolutely fixed for most elements. for example, the relative atomic mass of chlorine is 35.5 rather than a whole number. . Difference Chlorine Isotopes.
From www.alamy.com
chlorine chemical element isotopes atomic structure illustration Difference Chlorine Isotopes Unlike protons, the number of neutrons is not absolutely fixed for most elements. chlorine (cl) has isotopes with mass numbers ranging from 32 g mol −1 to 40 g mol −1. the primary difference between chlorine 35 and chlorine 37 lies in their atomic mass. these differing atoms of the same element are called isotopes. There are. Difference Chlorine Isotopes.
From www.savemyexams.co.uk
Isotopes (4.1.4) AQA GCSE Physics Revision Notes 2018 Save My Exams Difference Chlorine Isotopes chlorine (cl) has isotopes with mass numbers ranging from 32 g mol −1 to 40 g mol −1. natural chlorine is a mixture of two stable isotopes: the different types of chlorine are called. the primary difference between chlorine 35 and chlorine 37 lies in their atomic mass. chlorine consists of two isotopes, 35 cl. Difference Chlorine Isotopes.
From scienceprism.co.uk
How archaeologists use chemistry to find out about our past Science Prism Difference Chlorine Isotopes isotopes \(\ce{^35}cl\) and \(\ce{^37}cl\) are the two natural, stable isotopes of chlorine. Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. simultaneous detection of uranium isotopes and fluorine advances nuclear nonproliferation monitoring. (a) when a sample of elemental. All atoms of chlorine (cl) have 17 protons, but there are chlorine. This is because. Difference Chlorine Isotopes.
From www.chegg.com
Solved While the image above shows you only 3 isotopes of Difference Chlorine Isotopes There are two stable isotopes, 35. Four isotopes of helium (he) are shown in. the primary difference between chlorine 35 and chlorine 37 lies in their atomic mass. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. 13 rows chlorine ( 17 cl). Difference Chlorine Isotopes.
From www.differencebetween.com
Difference Between Chlorine and Chlorine Dioxide Compare the Difference Chlorine Isotopes chlorine consists of two isotopes, 35 cl and 37 cl, in approximately a 3:1 ratio. That of isotopes heavier than 37 cl. Chlorine 35 has an atomic. 13 rows chlorine ( 17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. this table shows information about naturally. Difference Chlorine Isotopes.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID5568233 Difference Chlorine Isotopes There are only two stable. There are two stable isotopes, 35. There are only two stable. Chlorine 35 has an atomic. isotopes \(\ce{^35}cl\) and \(\ce{^37}cl\) are the two natural, stable isotopes of chlorine. simultaneous detection of uranium isotopes and fluorine advances nuclear nonproliferation monitoring. the primary difference between chlorine 35 and chlorine 37 lies in their atomic. Difference Chlorine Isotopes.
From www.slideserve.com
PPT 1. Atomic Structure PowerPoint Presentation, free download ID Difference Chlorine Isotopes Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. This is because chlorine contains two. isotopes are atoms of the same element that differ in the amount of neutrons and atomic mass. Chlorine 35 has an atomic. (a) when a sample of elemental. chlorine (17 cl) has 25 isotopes, ranging from 28 cl. Difference Chlorine Isotopes.
From www.teachoo.com
Isotopes and Isobars Definition, Uses and Difference Teachoo Difference Chlorine Isotopes Unlike protons, the number of neutrons is not absolutely fixed for most elements. the primary decay mode of isotopes lighter than 35 cl is electron capture to isotopes of sulfur; this table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins,. This is because chlorine contains two. Four isotopes of helium (he). Difference Chlorine Isotopes.
From www.sciencephoto.com
Isotopes of chlorine, illustration Stock Image C028/6461 Science Difference Chlorine Isotopes Four isotopes of helium (he) are shown in. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. There are only two stable. This is because chlorine contains two. There are only two stable. Isotopes are forms of an element that have the same number of. Difference Chlorine Isotopes.
From www.aiophotoz.com
What Is An Isotope Examples Types And How To Identify An Isotope Difference Chlorine Isotopes Unlike protons, the number of neutrons is not absolutely fixed for most elements. Isotopes are forms of an element that have the same number of protons but different. for example, the relative atomic mass of chlorine is 35.5 rather than a whole number. this table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their. Difference Chlorine Isotopes.
From www.alamy.com
chlorine chemical element isotopes atomic structure illustration Difference Chlorine Isotopes There are two principal stable. Isotopes are forms of an element that have the same number of protons but different. That of isotopes heavier than 37 cl. chlorine (cl) has isotopes with mass numbers ranging from 32 g mol −1 to 40 g mol −1. simultaneous detection of uranium isotopes and fluorine advances nuclear nonproliferation monitoring. This is. Difference Chlorine Isotopes.
From www.teachoo.com
Isotopes and Isobars Definition, Uses and Difference Teachoo Difference Chlorine Isotopes There are only two stable. the different types of chlorine are called. Four isotopes of helium (he) are shown in. There are two stable isotopes, 35. All atoms of chlorine (cl) have 17 protons, but there are chlorine. simultaneous detection of uranium isotopes and fluorine advances nuclear nonproliferation monitoring. the primary decay mode of isotopes lighter than. Difference Chlorine Isotopes.
From www.doubtnut.com
(a) Give the schematic atomic structures of chlorine atom and chloride Difference Chlorine Isotopes the primary decay mode of isotopes lighter than 35 cl is electron capture to isotopes of sulfur; these differing atoms are called isotopes. Chlorine 35 has an atomic. for example, the relative atomic mass of chlorine is 35.5 rather than a whole number. isotopes \(\ce{^35}cl\) and \(\ce{^37}cl\) are the two natural, stable isotopes of chlorine. Unlike. Difference Chlorine Isotopes.
From www.slideserve.com
PPT Relative atomic mass PowerPoint Presentation ID5933036 Difference Chlorine Isotopes There are only two stable. There are two principal stable. isotopes are elements with different numbers of neutrons in the nucleus but the same number of protons. Chlorine 35 has an atomic. the primary difference between chlorine 35 and chlorine 37 lies in their atomic mass. this table shows information about naturally occuring isotopes, their atomic masses,. Difference Chlorine Isotopes.
From www.researchgate.net
Patterns of the molecular ion with multiple chlorine or bromine atoms Difference Chlorine Isotopes Unlike protons, the number of neutrons is not absolutely fixed for most elements. chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. these differing atoms of the same element are called isotopes. isotopes are elements with different numbers of neutrons in the nucleus but the. Difference Chlorine Isotopes.
From ar.inspiredpencil.com
Isotopes Of Chlorine Difference Chlorine Isotopes isotopes are elements with different numbers of neutrons in the nucleus but the same number of protons. Chlorine 35 has an atomic. There are only two stable. This is because chlorine contains two. Unlike protons, the number of neutrons is not absolutely fixed for most elements. This is because chlorine contains two. chlorine (17 cl) has 25 isotopes,. Difference Chlorine Isotopes.
From www.researchgate.net
Measured chlorine isotope ratios of (a) tetrachloroethylene (PCE) and Difference Chlorine Isotopes chlorine (cl) has isotopes with mass numbers ranging from 32 g mol −1 to 40 g mol −1. for example, the relative atomic mass of chlorine is 35.5 rather than a whole number. This is because chlorine contains two. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers,. Difference Chlorine Isotopes.
From www.youtube.com
Chlorine has two isotopes Cl35 and Cl37. Ratio present in nature is 31 Difference Chlorine Isotopes this table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins,. natural chlorine is a mixture of two stable isotopes: isotopes \(\ce{^35}cl\) and \(\ce{^37}cl\) are the two natural, stable isotopes of chlorine. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers,. Difference Chlorine Isotopes.
From dxosvaiso.blob.core.windows.net
Chlorine Element Number Of Isotopes at Calvin Petillo blog Difference Chlorine Isotopes Chlorine 35 has an atomic. for example, the relative atomic mass of chlorine is 35.5 rather than a whole number. these differing atoms of the same element are called isotopes. this table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins,. chlorine (cl) has isotopes with mass numbers ranging from. Difference Chlorine Isotopes.
From www.coursehero.com
[Solved] Chlorine has two naturally occurring isotopes Chlorine 35 Difference Chlorine Isotopes these differing atoms of the same element are called isotopes. There are only two stable. chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. Chlorine 35 has an atomic. 13 rows chlorine ( 17 cl) has 25 isotopes, ranging from 28 cl to 52 cl,. Difference Chlorine Isotopes.
From www.geochemicalperspectivesletters.org
Chlorine isotope ratios record magmatic brine assimilation during Difference Chlorine Isotopes isotopes are elements with different numbers of neutrons in the nucleus but the same number of protons. simultaneous detection of uranium isotopes and fluorine advances nuclear nonproliferation monitoring. 13 rows chlorine ( 17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. There are only two stable.. Difference Chlorine Isotopes.
From www.tpsearchtool.com
What Are Isotopes Definition Types Examples Images Difference Chlorine Isotopes All atoms of chlorine (cl) have 17 protons, but there are chlorine. There are two principal stable. natural chlorine is a mixture of two stable isotopes: for example, the relative atomic mass of chlorine is 35.5 rather than a whole number. isotopes \(\ce{^35}cl\) and \(\ce{^37}cl\) are the two natural, stable isotopes of chlorine. simultaneous detection of. Difference Chlorine Isotopes.
From www.differencebetween.com
Difference Between Chlorine 35 and 37 Compare the Difference Between Difference Chlorine Isotopes simultaneous detection of uranium isotopes and fluorine advances nuclear nonproliferation monitoring. the primary decay mode of isotopes lighter than 35 cl is electron capture to isotopes of sulfur; Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. chlorine consists of two isotopes, 35 cl and 37 cl, in approximately a 3:1 ratio.. Difference Chlorine Isotopes.