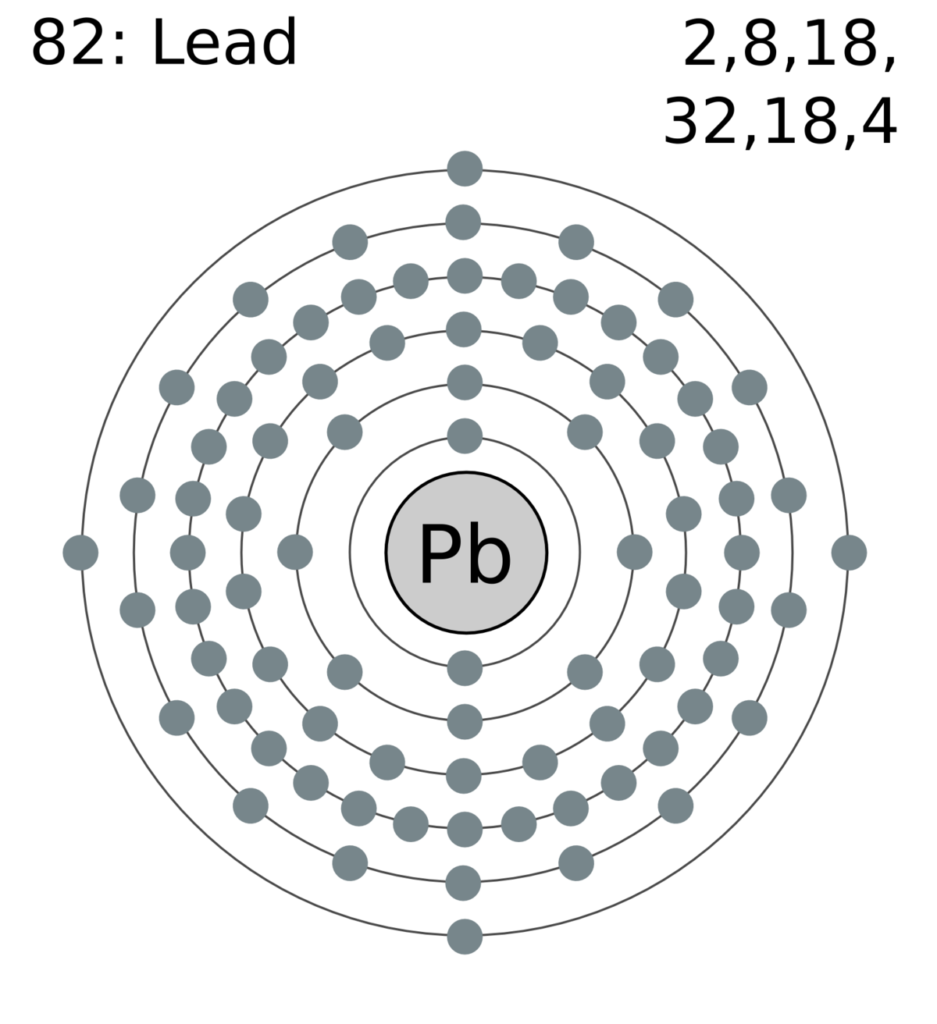
Lead Electron Valence . But for most of the transition. In chemistry and physics, a valence electron is an electron associated with an atom that can form a chemical bond and participate in a chemical reactions. The quest for the underlying causes of valence lead to the modern theories of chemical bonding, including lewis structures (1916), valence bond. Why are valence electrons important. They also determine the atom’s electronegativity, electron. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. The ground state electron configuration of ground state. The number of valence electrons determines the reactivity of the atom.
from periodictable.me
They also determine the atom’s electronegativity, electron. In chemistry and physics, a valence electron is an electron associated with an atom that can form a chemical bond and participate in a chemical reactions. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. But for most of the transition. The number of valence electrons determines the reactivity of the atom. Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. Why are valence electrons important. The quest for the underlying causes of valence lead to the modern theories of chemical bonding, including lewis structures (1916), valence bond. The ground state electron configuration of ground state.
Lead Valence Electrons Lead Valency (Pb) with Dot Diagram
Lead Electron Valence 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The quest for the underlying causes of valence lead to the modern theories of chemical bonding, including lewis structures (1916), valence bond. In chemistry and physics, a valence electron is an electron associated with an atom that can form a chemical bond and participate in a chemical reactions. The number of valence electrons determines the reactivity of the atom. But for most of the transition. Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. Why are valence electrons important. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The ground state electron configuration of ground state. They also determine the atom’s electronegativity, electron.
From
Lead Electron Valence In chemistry and physics, a valence electron is an electron associated with an atom that can form a chemical bond and participate in a chemical reactions. But for most of the transition. They also determine the atom’s electronegativity, electron. Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. The ground state electron configuration of ground state. The quest. Lead Electron Valence.
From
Lead Electron Valence In chemistry and physics, a valence electron is an electron associated with an atom that can form a chemical bond and participate in a chemical reactions. They also determine the atom’s electronegativity, electron. The ground state electron configuration of ground state. But for most of the transition. Why are valence electrons important. 93 rows you may assume the valences of. Lead Electron Valence.
From iperiodictable.com
How To Find an Valence Lead Electron Configuration (Pb) Lead Electron Valence But for most of the transition. Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. The ground state electron configuration of ground state. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. They also determine the atom’s electronegativity, electron. In chemistry and physics,. Lead Electron Valence.
From cabinet.matttroy.net
Lead Periodic Table Protons Neutrons And Electrons Matttroy Lead Electron Valence But for most of the transition. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The ground state electron configuration of ground state. They also determine the atom’s electronegativity, electron. Why are valence electrons important. In chemistry and physics, a valence electron is an electron. Lead Electron Valence.
From
Lead Electron Valence In chemistry and physics, a valence electron is an electron associated with an atom that can form a chemical bond and participate in a chemical reactions. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The ground state electron configuration of ground state. The quest. Lead Electron Valence.
From
Lead Electron Valence Why are valence electrons important. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. But for most of the transition. The quest for the underlying causes of valence lead to the modern theories of chemical bonding, including lewis structures (1916), valence bond. The number of. Lead Electron Valence.
From valenceelectrons.com
Complete Electron Configuration for Lead (Pb, Pb2+, Pb4+) Lead Electron Valence Why are valence electrons important. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The quest for the underlying causes of valence lead to the modern theories of chemical bonding, including lewis structures (1916), valence bond. The ground state electron configuration of ground state. In. Lead Electron Valence.
From www.slideserve.com
PPT Valence Electrons PowerPoint Presentation, free download ID Lead Electron Valence Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. The ground state electron configuration of ground state. Why are valence electrons important. But for most of the transition. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. In chemistry and physics, a valence. Lead Electron Valence.
From
Lead Electron Valence But for most of the transition. The ground state electron configuration of ground state. The number of valence electrons determines the reactivity of the atom. Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. They also determine the atom’s electronegativity, electron. In chemistry and physics, a valence electron is an electron associated with an atom that can form. Lead Electron Valence.
From valenceelectrons.com
Electron Configuration for Lead and Lead ions(Pb2+, Pb4+) Lead Electron Valence The quest for the underlying causes of valence lead to the modern theories of chemical bonding, including lewis structures (1916), valence bond. Why are valence electrons important. But for most of the transition. Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. 93 rows you may assume the valences of the chemical elements—the number of electrons with which. Lead Electron Valence.
From
Lead Electron Valence 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The ground state electron configuration of ground state. They also determine the atom’s electronegativity, electron. In chemistry and physics, a valence electron is an electron associated with an atom that can form a chemical bond and. Lead Electron Valence.
From www.youtube.com
Electron Configuration for Pb, Pb2+, and Pb4+ (Lead and Lead Ions Lead Electron Valence The quest for the underlying causes of valence lead to the modern theories of chemical bonding, including lewis structures (1916), valence bond. They also determine the atom’s electronegativity, electron. Why are valence electrons important. The ground state electron configuration of ground state. In chemistry and physics, a valence electron is an electron associated with an atom that can form a. Lead Electron Valence.
From www.webelements.com
Elements Periodic Table » Lead » properties of free atoms Lead Electron Valence Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. They also determine the atom’s electronegativity, electron. The quest for the underlying causes of valence lead to the modern theories of chemical bonding, including lewis structures (1916), valence bond. The number of valence electrons determines the reactivity of the atom. The ground state electron configuration of ground state. But. Lead Electron Valence.
From
Lead Electron Valence In chemistry and physics, a valence electron is an electron associated with an atom that can form a chemical bond and participate in a chemical reactions. Why are valence electrons important. The number of valence electrons determines the reactivity of the atom. They also determine the atom’s electronegativity, electron. 93 rows you may assume the valences of the chemical elements—the. Lead Electron Valence.
From
Lead Electron Valence The ground state electron configuration of ground state. Why are valence electrons important. In chemistry and physics, a valence electron is an electron associated with an atom that can form a chemical bond and participate in a chemical reactions. But for most of the transition. Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. They also determine the. Lead Electron Valence.
From
Lead Electron Valence They also determine the atom’s electronegativity, electron. The ground state electron configuration of ground state. But for most of the transition. Why are valence electrons important. The quest for the underlying causes of valence lead to the modern theories of chemical bonding, including lewis structures (1916), valence bond. The number of valence electrons determines the reactivity of the atom. 93. Lead Electron Valence.
From
Lead Electron Valence In chemistry and physics, a valence electron is an electron associated with an atom that can form a chemical bond and participate in a chemical reactions. But for most of the transition. Why are valence electrons important. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those. Lead Electron Valence.
From
Lead Electron Valence The quest for the underlying causes of valence lead to the modern theories of chemical bonding, including lewis structures (1916), valence bond. The number of valence electrons determines the reactivity of the atom. But for most of the transition. Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. Why are valence electrons important. In chemistry and physics, a. Lead Electron Valence.
From
Lead Electron Valence Why are valence electrons important. The ground state electron configuration of ground state. But for most of the transition. The number of valence electrons determines the reactivity of the atom. The quest for the underlying causes of valence lead to the modern theories of chemical bonding, including lewis structures (1916), valence bond. Lead atoms have 82 electrons and the shell. Lead Electron Valence.
From www.sciencefacts.net
Valence Electrons Definition, Location, Importance, and Diagram Lead Electron Valence In chemistry and physics, a valence electron is an electron associated with an atom that can form a chemical bond and participate in a chemical reactions. They also determine the atom’s electronegativity, electron. The number of valence electrons determines the reactivity of the atom. Why are valence electrons important. The quest for the underlying causes of valence lead to the. Lead Electron Valence.
From
Lead Electron Valence Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. Why are valence electrons important. In chemistry and physics, a valence electron is an electron associated with an atom that can form a chemical bond and participate in a chemical reactions. The quest for the underlying causes of valence lead to the modern theories of chemical bonding, including lewis. Lead Electron Valence.
From
Lead Electron Valence The ground state electron configuration of ground state. They also determine the atom’s electronegativity, electron. In chemistry and physics, a valence electron is an electron associated with an atom that can form a chemical bond and participate in a chemical reactions. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will. Lead Electron Valence.
From
Lead Electron Valence But for most of the transition. The quest for the underlying causes of valence lead to the modern theories of chemical bonding, including lewis structures (1916), valence bond. They also determine the atom’s electronegativity, electron. Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. 93 rows you may assume the valences of the chemical elements—the number of electrons. Lead Electron Valence.
From
Lead Electron Valence 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. In chemistry and physics, a valence electron is an electron associated with an atom that can form a chemical bond and participate in a chemical reactions. The quest for the underlying causes of valence lead to. Lead Electron Valence.
From
Lead Electron Valence The number of valence electrons determines the reactivity of the atom. The quest for the underlying causes of valence lead to the modern theories of chemical bonding, including lewis structures (1916), valence bond. But for most of the transition. They also determine the atom’s electronegativity, electron. In chemistry and physics, a valence electron is an electron associated with an atom. Lead Electron Valence.
From www.nuclear-power.com
Lead Electron Affinity Electronegativity Ionization Energy of Lead Electron Valence 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. But for most of the transition. In chemistry and physics, a valence electron is an electron associated with an atom that can form a chemical. Lead Electron Valence.
From
Lead Electron Valence They also determine the atom’s electronegativity, electron. But for most of the transition. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. In chemistry and physics, a valence electron is an electron associated with an atom that can form a chemical bond and participate in. Lead Electron Valence.
From valenceelectrons.com
Lead(Pb) Electron Configuration and Orbital Diagram Lead Electron Valence The quest for the underlying causes of valence lead to the modern theories of chemical bonding, including lewis structures (1916), valence bond. They also determine the atom’s electronegativity, electron. In chemistry and physics, a valence electron is an electron associated with an atom that can form a chemical bond and participate in a chemical reactions. 93 rows you may assume. Lead Electron Valence.
From
Lead Electron Valence The quest for the underlying causes of valence lead to the modern theories of chemical bonding, including lewis structures (1916), valence bond. They also determine the atom’s electronegativity, electron. In chemistry and physics, a valence electron is an electron associated with an atom that can form a chemical bond and participate in a chemical reactions. 93 rows you may assume. Lead Electron Valence.
From www.sciencephoto.com
Lead, atomic structure Stock Image C013/1639 Science Photo Library Lead Electron Valence But for most of the transition. Why are valence electrons important. The ground state electron configuration of ground state. They also determine the atom’s electronegativity, electron. The number of valence electrons determines the reactivity of the atom. In chemistry and physics, a valence electron is an electron associated with an atom that can form a chemical bond and participate in. Lead Electron Valence.
From
Lead Electron Valence Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The number of valence electrons determines the reactivity of the atom. But for most of the transition. The quest for the underlying causes of valence. Lead Electron Valence.
From www.youtube.com
Electron Configuration of Lead Pb Lesson YouTube Lead Electron Valence But for most of the transition. The ground state electron configuration of ground state. Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. They also determine the atom’s electronegativity, electron. The quest for the underlying causes of valence lead to the modern theories of chemical bonding, including lewis structures (1916), valence bond. In chemistry and physics, a valence. Lead Electron Valence.
From www.youtube.com
CHEMISTRY 101 Valence and core electrons YouTube Lead Electron Valence They also determine the atom’s electronegativity, electron. The ground state electron configuration of ground state. In chemistry and physics, a valence electron is an electron associated with an atom that can form a chemical bond and participate in a chemical reactions. The number of valence electrons determines the reactivity of the atom. 93 rows you may assume the valences of. Lead Electron Valence.
From howtomechatronics.com
What is Electric Charge and How Electricity Works How To Mechatronics Lead Electron Valence Why are valence electrons important. The quest for the underlying causes of valence lead to the modern theories of chemical bonding, including lewis structures (1916), valence bond. Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. In chemistry and physics, a valence electron is an electron associated with an atom that can form a chemical bond and participate. Lead Electron Valence.
From www.vectorstock.com
Atom symbol and electron of lead Royalty Free Vector Image Lead Electron Valence The quest for the underlying causes of valence lead to the modern theories of chemical bonding, including lewis structures (1916), valence bond. The number of valence electrons determines the reactivity of the atom. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. They also determine. Lead Electron Valence.