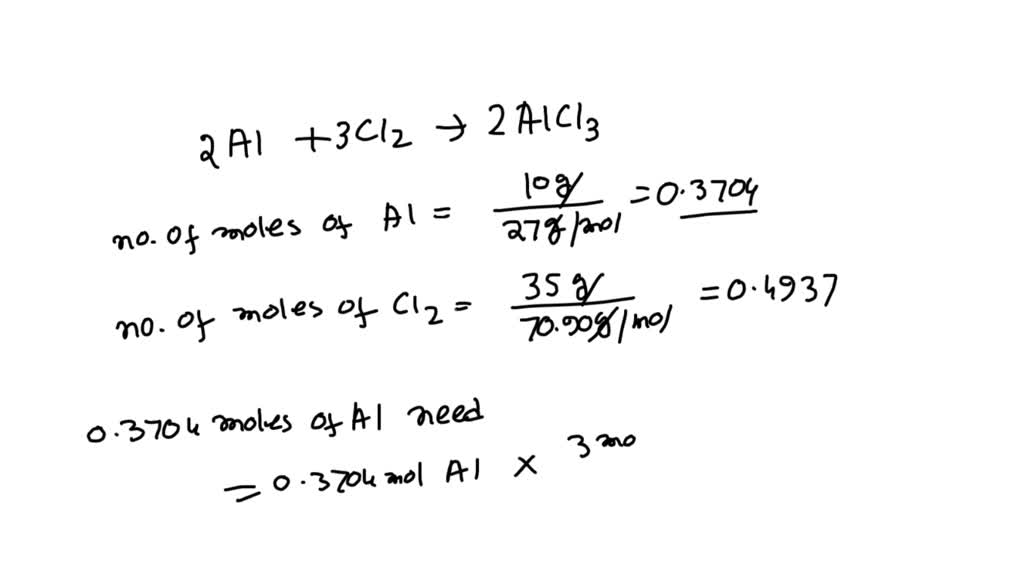Aluminum Reacts With Zinc Chloride . 3 aluminium reacts with zinc chloride because aluminium is more reactive than zinc. In a single displacement reaction, a. 9 rows recap the reactivity series of metals and how it's used to predict the outcomes of reactions with air, water and steam as. 2al + 3zncl₂ → 3zn + 2alcl₃. for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Al + zncl₂ → zn + alcl₃. when aluminum metal reacts with zinc chloride, the aluminum displaces zinc in the compound to form aluminum. al + zncl2 = zn + alcl3 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and. Al + zncl2 → zn + alcl3. Aluminum metal reacts with zinc chloride to produce zinc metal and aluminum chloride.
from www.numerade.com
when aluminum metal reacts with zinc chloride, the aluminum displaces zinc in the compound to form aluminum. In a single displacement reaction, a. 9 rows recap the reactivity series of metals and how it's used to predict the outcomes of reactions with air, water and steam as. Aluminum metal reacts with zinc chloride to produce zinc metal and aluminum chloride. Al + zncl₂ → zn + alcl₃. for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Al + zncl2 → zn + alcl3. 2al + 3zncl₂ → 3zn + 2alcl₃. 3 aluminium reacts with zinc chloride because aluminium is more reactive than zinc. al + zncl2 = zn + alcl3 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and.
SOLVED 10.g of aluminum reacts with 35. grams of chlorine gas to
Aluminum Reacts With Zinc Chloride Aluminum metal reacts with zinc chloride to produce zinc metal and aluminum chloride. for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. when aluminum metal reacts with zinc chloride, the aluminum displaces zinc in the compound to form aluminum. In a single displacement reaction, a. Aluminum metal reacts with zinc chloride to produce zinc metal and aluminum chloride. Al + zncl₂ → zn + alcl₃. 3 aluminium reacts with zinc chloride because aluminium is more reactive than zinc. Al + zncl2 → zn + alcl3. 2al + 3zncl₂ → 3zn + 2alcl₃. 9 rows recap the reactivity series of metals and how it's used to predict the outcomes of reactions with air, water and steam as. al + zncl2 = zn + alcl3 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and.
From www.numerade.com
SOLVEDWhat are the products formed when chlorine reacts with (i Aluminum Reacts With Zinc Chloride In a single displacement reaction, a. Al + zncl₂ → zn + alcl₃. Aluminum metal reacts with zinc chloride to produce zinc metal and aluminum chloride. 3 aluminium reacts with zinc chloride because aluminium is more reactive than zinc. 2al + 3zncl₂ → 3zn + 2alcl₃. when aluminum metal reacts with zinc chloride, the aluminum displaces zinc in. Aluminum Reacts With Zinc Chloride.
From www.numerade.com
SOLVED Aluminum metal reacts with zinc chloride to produce zinc metal Aluminum Reacts With Zinc Chloride 3 aluminium reacts with zinc chloride because aluminium is more reactive than zinc. Aluminum metal reacts with zinc chloride to produce zinc metal and aluminum chloride. Al + zncl2 → zn + alcl3. al + zncl2 = zn + alcl3 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and. In a single displacement. Aluminum Reacts With Zinc Chloride.
From www.nagwa.com
Question Video Identifying the Equation That Describes What Happens to Aluminum Reacts With Zinc Chloride Al + zncl₂ → zn + alcl₃. 9 rows recap the reactivity series of metals and how it's used to predict the outcomes of reactions with air, water and steam as. Al + zncl2 → zn + alcl3. al + zncl2 = zn + alcl3 is a single displacement (substitution) reaction where two moles of solid aluminium [al]. Aluminum Reacts With Zinc Chloride.
From www.toppr.com
5. What happens when a] Zinc reacts with Copper Sulphate solution. b Aluminum Reacts With Zinc Chloride Al + zncl2 → zn + alcl3. Aluminum metal reacts with zinc chloride to produce zinc metal and aluminum chloride. al + zncl2 = zn + alcl3 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and. 2al + 3zncl₂ → 3zn + 2alcl₃. Al + zncl₂ → zn + alcl₃. 9 rows recap. Aluminum Reacts With Zinc Chloride.
From www.chegg.com
Solved Aluminum reacts with chlorine gas to form aluminum Aluminum Reacts With Zinc Chloride Al + zncl2 → zn + alcl3. when aluminum metal reacts with zinc chloride, the aluminum displaces zinc in the compound to form aluminum. In a single displacement reaction, a. Aluminum metal reacts with zinc chloride to produce zinc metal and aluminum chloride. for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. . Aluminum Reacts With Zinc Chloride.
From slideplayer.com
Chemical Reactions. ppt download Aluminum Reacts With Zinc Chloride al + zncl2 = zn + alcl3 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and. for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. 2al + 3zncl₂ → 3zn + 2alcl₃. In a single displacement reaction, a. Aluminum metal reacts with zinc chloride to produce zinc metal. Aluminum Reacts With Zinc Chloride.
From www.numerade.com
SOLVED Aluminum reacts with chlorine gas to form aluminum chloride via Aluminum Reacts With Zinc Chloride al + zncl2 = zn + alcl3 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and. In a single displacement reaction, a. Al + zncl2 → zn + alcl3. for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. when aluminum metal reacts with zinc chloride, the aluminum. Aluminum Reacts With Zinc Chloride.
From www.numerade.com
SOLVED Aluminum reacts with chlorine gas to produce aluminum chloride Aluminum Reacts With Zinc Chloride 9 rows recap the reactivity series of metals and how it's used to predict the outcomes of reactions with air, water and steam as. Aluminum metal reacts with zinc chloride to produce zinc metal and aluminum chloride. al + zncl2 = zn + alcl3 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and.. Aluminum Reacts With Zinc Chloride.
From express.adobe.com
Zinc and Copper Chloride Aluminum Reacts With Zinc Chloride In a single displacement reaction, a. for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Aluminum metal reacts with zinc chloride to produce zinc metal and aluminum chloride. al + zncl2 = zn + alcl3 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and. 3 aluminium reacts. Aluminum Reacts With Zinc Chloride.
From www.toppr.com
The reaction of benzene with chlorine in the presence of iron gives Aluminum Reacts With Zinc Chloride al + zncl2 = zn + alcl3 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and. for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. 2al + 3zncl₂ → 3zn + 2alcl₃. Al + zncl₂ → zn + alcl₃. 3 aluminium reacts with zinc chloride because aluminium. Aluminum Reacts With Zinc Chloride.
From fyombzhup.blob.core.windows.net
Barium Chloride Symbol at Mary Masse blog Aluminum Reacts With Zinc Chloride when aluminum metal reacts with zinc chloride, the aluminum displaces zinc in the compound to form aluminum. 2al + 3zncl₂ → 3zn + 2alcl₃. 3 aluminium reacts with zinc chloride because aluminium is more reactive than zinc. In a single displacement reaction, a. Al + zncl₂ → zn + alcl₃. Al + zncl2 → zn + alcl3. . Aluminum Reacts With Zinc Chloride.
From www.teachoo.com
Assertion (A) When zinc is added to dilute hydrochloric acid, hydro Aluminum Reacts With Zinc Chloride 3 aluminium reacts with zinc chloride because aluminium is more reactive than zinc. for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Aluminum metal reacts with zinc chloride to produce zinc metal and aluminum chloride. 2al + 3zncl₂ → 3zn + 2alcl₃. In a single displacement reaction, a. 9 rows recap the. Aluminum Reacts With Zinc Chloride.
From www.numerade.com
SOLVED Ferric chloride is formed by the reaction of iron and chlorine Aluminum Reacts With Zinc Chloride Al + zncl₂ → zn + alcl₃. Al + zncl2 → zn + alcl3. 3 aluminium reacts with zinc chloride because aluminium is more reactive than zinc. Aluminum metal reacts with zinc chloride to produce zinc metal and aluminum chloride. 9 rows recap the reactivity series of metals and how it's used to predict the outcomes of reactions. Aluminum Reacts With Zinc Chloride.
From www.slideserve.com
PPT Quiz PowerPoint Presentation, free download ID3201515 Aluminum Reacts With Zinc Chloride 9 rows recap the reactivity series of metals and how it's used to predict the outcomes of reactions with air, water and steam as. al + zncl2 = zn + alcl3 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and. for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and. Aluminum Reacts With Zinc Chloride.
From www.youtube.com
Aluminum and Copper (II) Chloride Reaction YouTube Aluminum Reacts With Zinc Chloride 9 rows recap the reactivity series of metals and how it's used to predict the outcomes of reactions with air, water and steam as. Aluminum metal reacts with zinc chloride to produce zinc metal and aluminum chloride. for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. In a single displacement reaction, a. . Aluminum Reacts With Zinc Chloride.
From www.youtube.com
Aluminium reacts with chlorine gas to form aluminium chloride via the Aluminum Reacts With Zinc Chloride 3 aluminium reacts with zinc chloride because aluminium is more reactive than zinc. Aluminum metal reacts with zinc chloride to produce zinc metal and aluminum chloride. Al + zncl₂ → zn + alcl₃. 2al + 3zncl₂ → 3zn + 2alcl₃. In a single displacement reaction, a. 9 rows recap the reactivity series of metals and how it's used. Aluminum Reacts With Zinc Chloride.
From www.coursehero.com
[Solved] Aluminum reacts with chlorine gas to produce aluminum chloride Aluminum Reacts With Zinc Chloride for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. 9 rows recap the reactivity series of metals and how it's used to predict the outcomes of reactions with air, water and steam as. Al + zncl₂ → zn + alcl₃. 3 aluminium reacts with zinc chloride because aluminium is more reactive than. Aluminum Reacts With Zinc Chloride.
From www.nagwa.com
Question Video Describing the Correct Symbol Equation for the Reaction Aluminum Reacts With Zinc Chloride al + zncl2 = zn + alcl3 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and. Al + zncl₂ → zn + alcl₃. 2al + 3zncl₂ → 3zn + 2alcl₃. when aluminum metal reacts with zinc chloride, the aluminum displaces zinc in the compound to form aluminum. Aluminum metal reacts with zinc chloride. Aluminum Reacts With Zinc Chloride.
From www.youtube.com
Reaction of Chlorine with Aluminium YouTube Aluminum Reacts With Zinc Chloride 3 aluminium reacts with zinc chloride because aluminium is more reactive than zinc. for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. In a single displacement reaction, a. Al + zncl₂ → zn + alcl₃. Al + zncl2 → zn + alcl3. Aluminum metal reacts with zinc chloride to produce zinc metal and. Aluminum Reacts With Zinc Chloride.
From www.numerade.com
SOLVED Aluminum reacts with chlorine to produce aluminum chloride. If Aluminum Reacts With Zinc Chloride 9 rows recap the reactivity series of metals and how it's used to predict the outcomes of reactions with air, water and steam as. when aluminum metal reacts with zinc chloride, the aluminum displaces zinc in the compound to form aluminum. 2al + 3zncl₂ → 3zn + 2alcl₃. In a single displacement reaction, a. al + zncl2. Aluminum Reacts With Zinc Chloride.
From www.numerade.com
SOLVED Write a balanced equation for the redox reactions. (a) The Aluminum Reacts With Zinc Chloride al + zncl2 = zn + alcl3 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and. 9 rows recap the reactivity series of metals and how it's used to predict the outcomes of reactions with air, water and steam as. 3 aluminium reacts with zinc chloride because aluminium is more reactive than. Aluminum Reacts With Zinc Chloride.
From lessonlibthrashings.z22.web.core.windows.net
Zinc And Acid Reaction Aluminum Reacts With Zinc Chloride al + zncl2 = zn + alcl3 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and. Al + zncl2 → zn + alcl3. 3 aluminium reacts with zinc chloride because aluminium is more reactive than zinc. Al + zncl₂ → zn + alcl₃. for example, zinc metal reacts with hydrochloric acid, producing. Aluminum Reacts With Zinc Chloride.
From www.chegg.com
Solved Ceanna Tra ion 5 Name 1) 1) How many grams of Aluminum Reacts With Zinc Chloride Al + zncl₂ → zn + alcl₃. 2al + 3zncl₂ → 3zn + 2alcl₃. al + zncl2 = zn + alcl3 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and. In a single displacement reaction, a. for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Al + zncl2. Aluminum Reacts With Zinc Chloride.
From www.numerade.com
SOLVEDWhat are the products formed when chlorine reacts with (i Aluminum Reacts With Zinc Chloride 2al + 3zncl₂ → 3zn + 2alcl₃. for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. al + zncl2 = zn + alcl3 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and. Aluminum metal reacts with zinc chloride to produce zinc metal and aluminum chloride. Al + zncl2. Aluminum Reacts With Zinc Chloride.
From www.youtube.com
Reaction of Zinc and Hydrochloric acid YouTube Aluminum Reacts With Zinc Chloride 9 rows recap the reactivity series of metals and how it's used to predict the outcomes of reactions with air, water and steam as. for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Aluminum metal reacts with zinc chloride to produce zinc metal and aluminum chloride. In a single displacement reaction, a. . Aluminum Reacts With Zinc Chloride.
From www.chegg.com
Solved Aluminum reacts with chlorine gas to form aluminum Aluminum Reacts With Zinc Chloride 2al + 3zncl₂ → 3zn + 2alcl₃. when aluminum metal reacts with zinc chloride, the aluminum displaces zinc in the compound to form aluminum. 9 rows recap the reactivity series of metals and how it's used to predict the outcomes of reactions with air, water and steam as. al + zncl2 = zn + alcl3 is a. Aluminum Reacts With Zinc Chloride.
From www.slideserve.com
PPT Chapter 8 Review Topics PowerPoint Presentation, free download Aluminum Reacts With Zinc Chloride al + zncl2 = zn + alcl3 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and. Al + zncl2 → zn + alcl3. for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. when aluminum metal reacts with zinc chloride, the aluminum displaces zinc in the compound to. Aluminum Reacts With Zinc Chloride.
From www.youtube.com
Reaction of Water with Aluminium Chloride YouTube Aluminum Reacts With Zinc Chloride Aluminum metal reacts with zinc chloride to produce zinc metal and aluminum chloride. Al + zncl₂ → zn + alcl₃. 3 aluminium reacts with zinc chloride because aluminium is more reactive than zinc. Al + zncl2 → zn + alcl3. In a single displacement reaction, a. 9 rows recap the reactivity series of metals and how it's used. Aluminum Reacts With Zinc Chloride.
From express.adobe.com
Zinc and Copper Chloride Reaction Aluminum Reacts With Zinc Chloride 2al + 3zncl₂ → 3zn + 2alcl₃. when aluminum metal reacts with zinc chloride, the aluminum displaces zinc in the compound to form aluminum. 3 aluminium reacts with zinc chloride because aluminium is more reactive than zinc. 9 rows recap the reactivity series of metals and how it's used to predict the outcomes of reactions with air,. Aluminum Reacts With Zinc Chloride.
From www.youtube.com
What happens when AlCl3, Aluminium chloride & Ca3N2, Calcium nitride Aluminum Reacts With Zinc Chloride 2al + 3zncl₂ → 3zn + 2alcl₃. Aluminum metal reacts with zinc chloride to produce zinc metal and aluminum chloride. when aluminum metal reacts with zinc chloride, the aluminum displaces zinc in the compound to form aluminum. for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Al + zncl₂ → zn + alcl₃.. Aluminum Reacts With Zinc Chloride.
From socratic.org
How do you write the equation for this reaction Aluminum bromide and Aluminum Reacts With Zinc Chloride In a single displacement reaction, a. al + zncl2 = zn + alcl3 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and. Aluminum metal reacts with zinc chloride to produce zinc metal and aluminum chloride. for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Al + zncl₂ →. Aluminum Reacts With Zinc Chloride.
From giofztxmx.blob.core.windows.net
Zinc Reacts With Hydrochloric Acid Formula at Tomas Greenwood blog Aluminum Reacts With Zinc Chloride 9 rows recap the reactivity series of metals and how it's used to predict the outcomes of reactions with air, water and steam as. for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. In a single displacement reaction, a. Aluminum metal reacts with zinc chloride to produce zinc metal and aluminum chloride. . Aluminum Reacts With Zinc Chloride.
From askfilo.com
Problem 3 Aluminum reacts with chlorine gas to form aluminum chloride v.. Aluminum Reacts With Zinc Chloride Aluminum metal reacts with zinc chloride to produce zinc metal and aluminum chloride. for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. 9 rows recap the reactivity series of metals and how it's used to predict the outcomes of reactions with air, water and steam as. al + zncl2 = zn +. Aluminum Reacts With Zinc Chloride.
From www.toppr.com
Translate the following statements into chemical equations and then Aluminum Reacts With Zinc Chloride when aluminum metal reacts with zinc chloride, the aluminum displaces zinc in the compound to form aluminum. Aluminum metal reacts with zinc chloride to produce zinc metal and aluminum chloride. al + zncl2 = zn + alcl3 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and. 2al + 3zncl₂ → 3zn + 2alcl₃.. Aluminum Reacts With Zinc Chloride.
From www.numerade.com
SOLVED 10.g of aluminum reacts with 35. grams of chlorine gas to Aluminum Reacts With Zinc Chloride 9 rows recap the reactivity series of metals and how it's used to predict the outcomes of reactions with air, water and steam as. 3 aluminium reacts with zinc chloride because aluminium is more reactive than zinc. when aluminum metal reacts with zinc chloride, the aluminum displaces zinc in the compound to form aluminum. al +. Aluminum Reacts With Zinc Chloride.