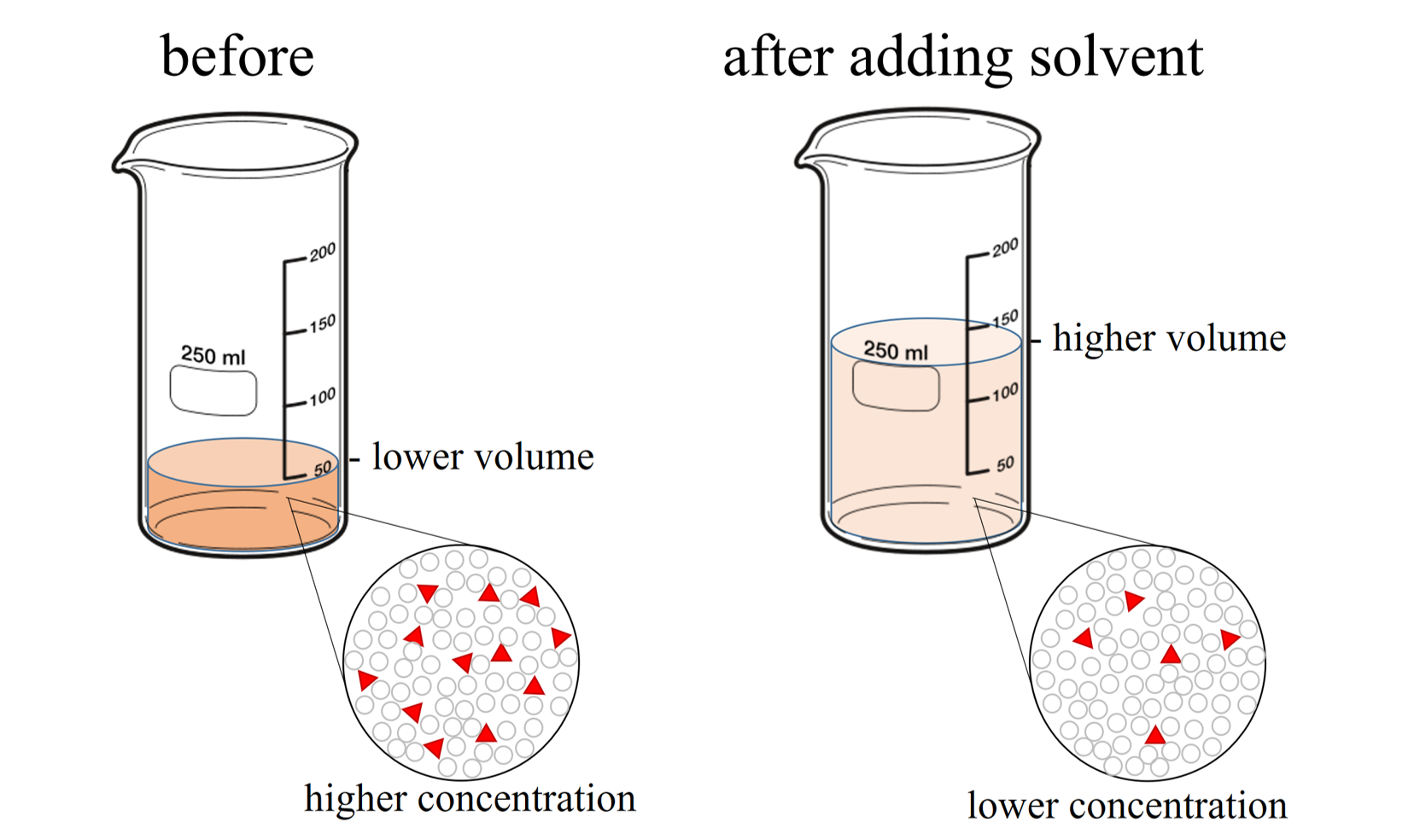
Dilute Solutions Of Alkali Hydroxides . a familiar reaction is that between magnesium and dilute sulfuric acid, producing hydrogen gas and a colorless. model description of the equivalent electroconductivity of aqueous solutions of alkali metal hydroxides over a wide range. having validated the simulation method for naoh for 17m concentration, we now present comprehensive structural and. it is possible in many instances to prepare lower normalities accurately by making an exact dilution of a stronger solution. Describe their reactions with water, oxygen and dilute acids,. where a reagent solution is described using an expression such as “hydrochloric acid (10 g/l hcl)”, the solution is prepared by an. revise group 2 reactions for your a level chemistry course. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution.
from chem.libretexts.org
Describe their reactions with water, oxygen and dilute acids,. where a reagent solution is described using an expression such as “hydrochloric acid (10 g/l hcl)”, the solution is prepared by an. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. a familiar reaction is that between magnesium and dilute sulfuric acid, producing hydrogen gas and a colorless. it is possible in many instances to prepare lower normalities accurately by making an exact dilution of a stronger solution. model description of the equivalent electroconductivity of aqueous solutions of alkali metal hydroxides over a wide range. having validated the simulation method for naoh for 17m concentration, we now present comprehensive structural and. revise group 2 reactions for your a level chemistry course.
14.7 Solution Dilution Chemistry LibreTexts
Dilute Solutions Of Alkali Hydroxides where a reagent solution is described using an expression such as “hydrochloric acid (10 g/l hcl)”, the solution is prepared by an. having validated the simulation method for naoh for 17m concentration, we now present comprehensive structural and. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. it is possible in many instances to prepare lower normalities accurately by making an exact dilution of a stronger solution. model description of the equivalent electroconductivity of aqueous solutions of alkali metal hydroxides over a wide range. a familiar reaction is that between magnesium and dilute sulfuric acid, producing hydrogen gas and a colorless. revise group 2 reactions for your a level chemistry course. Describe their reactions with water, oxygen and dilute acids,. where a reagent solution is described using an expression such as “hydrochloric acid (10 g/l hcl)”, the solution is prepared by an.
From www.youtube.com
Oxides and hydroxides of alkali metals / Alkali & alkaline earth metals Dilute Solutions Of Alkali Hydroxides Describe their reactions with water, oxygen and dilute acids,. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. it is possible in many instances to prepare lower normalities accurately by making an exact dilution of a stronger solution. model description of the equivalent electroconductivity of aqueous solutions. Dilute Solutions Of Alkali Hydroxides.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilute Solutions Of Alkali Hydroxides model description of the equivalent electroconductivity of aqueous solutions of alkali metal hydroxides over a wide range. having validated the simulation method for naoh for 17m concentration, we now present comprehensive structural and. Describe their reactions with water, oxygen and dilute acids,. a familiar reaction is that between magnesium and dilute sulfuric acid, producing hydrogen gas and. Dilute Solutions Of Alkali Hydroxides.
From astonishingceiyrs.blogspot.com
Weak Alkali Examples astonishingceiyrs Dilute Solutions Of Alkali Hydroxides having validated the simulation method for naoh for 17m concentration, we now present comprehensive structural and. it is possible in many instances to prepare lower normalities accurately by making an exact dilution of a stronger solution. model description of the equivalent electroconductivity of aqueous solutions of alkali metal hydroxides over a wide range. a dilute solution. Dilute Solutions Of Alkali Hydroxides.
From slidetodoc.com
Groups 1 and 2 Compounds 09 November 2020 Dilute Solutions Of Alkali Hydroxides a familiar reaction is that between magnesium and dilute sulfuric acid, producing hydrogen gas and a colorless. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. having validated the simulation method for naoh for 17m concentration, we now present comprehensive structural and. it is possible in. Dilute Solutions Of Alkali Hydroxides.
From www.youtube.com
Aqueous, Dilute And Concentrated Solution, General Science Lecture Dilute Solutions Of Alkali Hydroxides it is possible in many instances to prepare lower normalities accurately by making an exact dilution of a stronger solution. where a reagent solution is described using an expression such as “hydrochloric acid (10 g/l hcl)”, the solution is prepared by an. a familiar reaction is that between magnesium and dilute sulfuric acid, producing hydrogen gas and. Dilute Solutions Of Alkali Hydroxides.
From www.pinterest.co.uk
Alkali metals react with water to produce hydroxides. Alkali metal Dilute Solutions Of Alkali Hydroxides revise group 2 reactions for your a level chemistry course. a familiar reaction is that between magnesium and dilute sulfuric acid, producing hydrogen gas and a colorless. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. having validated the simulation method for naoh for 17m concentration,. Dilute Solutions Of Alkali Hydroxides.
From slideplayer.com
Alkali Metals Noadswood Science, ppt download Dilute Solutions Of Alkali Hydroxides a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. model description of the equivalent electroconductivity of aqueous solutions of alkali metal hydroxides over a wide range. a familiar reaction is that between magnesium and dilute sulfuric acid, producing hydrogen gas and a colorless. Describe their reactions with. Dilute Solutions Of Alkali Hydroxides.
From byjus.com
40.Why does the solubility of alkaline earth metal hydroxides in water Dilute Solutions Of Alkali Hydroxides revise group 2 reactions for your a level chemistry course. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. model description of the equivalent electroconductivity of aqueous solutions of alkali metal hydroxides over a wide range. it is possible in many instances to prepare lower normalities. Dilute Solutions Of Alkali Hydroxides.
From www.linstitute.net
CIE A Level Chemistry复习笔记2.2.2 Reactions of Group 2 Oxides, Hydroxides Dilute Solutions Of Alkali Hydroxides it is possible in many instances to prepare lower normalities accurately by making an exact dilution of a stronger solution. model description of the equivalent electroconductivity of aqueous solutions of alkali metal hydroxides over a wide range. having validated the simulation method for naoh for 17m concentration, we now present comprehensive structural and. a dilute solution. Dilute Solutions Of Alkali Hydroxides.
From slideplayer.com
Qualitative Analysis Dr. VALMIK KAPASE D. P ppt download Dilute Solutions Of Alkali Hydroxides a familiar reaction is that between magnesium and dilute sulfuric acid, producing hydrogen gas and a colorless. having validated the simulation method for naoh for 17m concentration, we now present comprehensive structural and. revise group 2 reactions for your a level chemistry course. Describe their reactions with water, oxygen and dilute acids,. a dilute solution is. Dilute Solutions Of Alkali Hydroxides.
From giovillwh.blob.core.windows.net
Dilution Factor Cell Counting at Richard Lockett blog Dilute Solutions Of Alkali Hydroxides model description of the equivalent electroconductivity of aqueous solutions of alkali metal hydroxides over a wide range. a familiar reaction is that between magnesium and dilute sulfuric acid, producing hydrogen gas and a colorless. revise group 2 reactions for your a level chemistry course. it is possible in many instances to prepare lower normalities accurately by. Dilute Solutions Of Alkali Hydroxides.
From www.slideserve.com
PPT The effect of dilution on the pH of an acid PowerPoint Dilute Solutions Of Alkali Hydroxides Describe their reactions with water, oxygen and dilute acids,. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. model description of the equivalent electroconductivity of aqueous solutions of alkali metal hydroxides over a wide range. revise group 2 reactions for your a level chemistry course. a. Dilute Solutions Of Alkali Hydroxides.
From www.numerade.com
SOLVEDWhen alkaline earth metals dissolve in ammonia, they form Dilute Solutions Of Alkali Hydroxides model description of the equivalent electroconductivity of aqueous solutions of alkali metal hydroxides over a wide range. it is possible in many instances to prepare lower normalities accurately by making an exact dilution of a stronger solution. revise group 2 reactions for your a level chemistry course. having validated the simulation method for naoh for 17m. Dilute Solutions Of Alkali Hydroxides.
From byjus.com
4 why the alkaline earth metal oxide form sparingly soluble hydroxides Dilute Solutions Of Alkali Hydroxides revise group 2 reactions for your a level chemistry course. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. a familiar reaction is that between magnesium and dilute sulfuric acid, producing hydrogen gas and a colorless. model description of the equivalent electroconductivity of aqueous solutions of. Dilute Solutions Of Alkali Hydroxides.
From pubs.acs.org
THE CONDUCTANCE OF DILUTE AQUEOUS SOLUTIONS OF THE ALKALI HYDROXIDES AT Dilute Solutions Of Alkali Hydroxides a familiar reaction is that between magnesium and dilute sulfuric acid, producing hydrogen gas and a colorless. it is possible in many instances to prepare lower normalities accurately by making an exact dilution of a stronger solution. having validated the simulation method for naoh for 17m concentration, we now present comprehensive structural and. model description of. Dilute Solutions Of Alkali Hydroxides.
From www.teachoo.com
Reactions of Acids and Bases Full list (with Examples) Teachoo Dilute Solutions Of Alkali Hydroxides it is possible in many instances to prepare lower normalities accurately by making an exact dilution of a stronger solution. revise group 2 reactions for your a level chemistry course. model description of the equivalent electroconductivity of aqueous solutions of alkali metal hydroxides over a wide range. a dilute solution is one in which there is. Dilute Solutions Of Alkali Hydroxides.
From www.slideserve.com
PPT Acids, Bases and Alkalis PowerPoint Presentation, free download Dilute Solutions Of Alkali Hydroxides it is possible in many instances to prepare lower normalities accurately by making an exact dilution of a stronger solution. having validated the simulation method for naoh for 17m concentration, we now present comprehensive structural and. a familiar reaction is that between magnesium and dilute sulfuric acid, producing hydrogen gas and a colorless. model description of. Dilute Solutions Of Alkali Hydroxides.
From www.slideserve.com
PPT The effect of dilution on the pH of an acid PowerPoint Dilute Solutions Of Alkali Hydroxides a familiar reaction is that between magnesium and dilute sulfuric acid, producing hydrogen gas and a colorless. revise group 2 reactions for your a level chemistry course. model description of the equivalent electroconductivity of aqueous solutions of alkali metal hydroxides over a wide range. Describe their reactions with water, oxygen and dilute acids,. where a reagent. Dilute Solutions Of Alkali Hydroxides.
From chemistry-europe.onlinelibrary.wiley.com
Chemical Absorption of CO2 Enhanced by Solutions of Alkali Hydroxides Dilute Solutions Of Alkali Hydroxides where a reagent solution is described using an expression such as “hydrochloric acid (10 g/l hcl)”, the solution is prepared by an. a familiar reaction is that between magnesium and dilute sulfuric acid, producing hydrogen gas and a colorless. model description of the equivalent electroconductivity of aqueous solutions of alkali metal hydroxides over a wide range. . Dilute Solutions Of Alkali Hydroxides.
From studymind.co.uk
Group 1 Reactions (GCSE Chemistry) Study Mind Dilute Solutions Of Alkali Hydroxides Describe their reactions with water, oxygen and dilute acids,. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. where a reagent solution is described using an expression such as “hydrochloric acid (10 g/l hcl)”, the solution is prepared by an. model description of the equivalent electroconductivity of. Dilute Solutions Of Alkali Hydroxides.
From 18thtimelucky.wordpress.com
Synthesis of alkali metal hydroxides via electrolysis 18thTimeLucky Dilute Solutions Of Alkali Hydroxides Describe their reactions with water, oxygen and dilute acids,. having validated the simulation method for naoh for 17m concentration, we now present comprehensive structural and. model description of the equivalent electroconductivity of aqueous solutions of alkali metal hydroxides over a wide range. a dilute solution is one in which there is a relatively small amount of solute. Dilute Solutions Of Alkali Hydroxides.
From slideplayer.com
Topic 9 ReactionsofAcids. Titrations Burette with acid solution e.g Dilute Solutions Of Alkali Hydroxides a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. where a reagent solution is described using an expression such as “hydrochloric acid (10 g/l hcl)”, the solution is prepared by an. having validated the simulation method for naoh for 17m concentration, we now present comprehensive structural and.. Dilute Solutions Of Alkali Hydroxides.
From slideplayer.com
Reaction of alkali metals with water ppt download Dilute Solutions Of Alkali Hydroxides it is possible in many instances to prepare lower normalities accurately by making an exact dilution of a stronger solution. a familiar reaction is that between magnesium and dilute sulfuric acid, producing hydrogen gas and a colorless. Describe their reactions with water, oxygen and dilute acids,. model description of the equivalent electroconductivity of aqueous solutions of alkali. Dilute Solutions Of Alkali Hydroxides.
From www.numerade.com
SOLVED solution containing mixture of metal cations was treated as Dilute Solutions Of Alkali Hydroxides model description of the equivalent electroconductivity of aqueous solutions of alkali metal hydroxides over a wide range. Describe their reactions with water, oxygen and dilute acids,. a familiar reaction is that between magnesium and dilute sulfuric acid, producing hydrogen gas and a colorless. where a reagent solution is described using an expression such as “hydrochloric acid (10. Dilute Solutions Of Alkali Hydroxides.
From slideplayer.com
Chemical reactions in aqueous solution ppt download Dilute Solutions Of Alkali Hydroxides where a reagent solution is described using an expression such as “hydrochloric acid (10 g/l hcl)”, the solution is prepared by an. revise group 2 reactions for your a level chemistry course. it is possible in many instances to prepare lower normalities accurately by making an exact dilution of a stronger solution. a dilute solution is. Dilute Solutions Of Alkali Hydroxides.
From byjus.com
4 why the alkaline earth metal oxide form sparingly soluble hydroxides Dilute Solutions Of Alkali Hydroxides a familiar reaction is that between magnesium and dilute sulfuric acid, producing hydrogen gas and a colorless. revise group 2 reactions for your a level chemistry course. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Describe their reactions with water, oxygen and dilute acids,. having. Dilute Solutions Of Alkali Hydroxides.
From www.youtube.com
Solutions by Dilution and Factors Affecting Rate of Solubility YouTube Dilute Solutions Of Alkali Hydroxides it is possible in many instances to prepare lower normalities accurately by making an exact dilution of a stronger solution. a familiar reaction is that between magnesium and dilute sulfuric acid, producing hydrogen gas and a colorless. revise group 2 reactions for your a level chemistry course. a dilute solution is one in which there is. Dilute Solutions Of Alkali Hydroxides.
From www.youtube.com
Properties of Alkali and Alkaline Earth Metals Hydroxides, Chemistry Dilute Solutions Of Alkali Hydroxides a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. model description of the equivalent electroconductivity of aqueous solutions of alkali metal hydroxides over a wide range. where a reagent solution is described using an expression such as “hydrochloric acid (10 g/l hcl)”, the solution is prepared by. Dilute Solutions Of Alkali Hydroxides.
From www.numerade.com
SOLVED A solution containing a mixture of metal cations was treated as Dilute Solutions Of Alkali Hydroxides Describe their reactions with water, oxygen and dilute acids,. revise group 2 reactions for your a level chemistry course. it is possible in many instances to prepare lower normalities accurately by making an exact dilution of a stronger solution. where a reagent solution is described using an expression such as “hydrochloric acid (10 g/l hcl)”, the solution. Dilute Solutions Of Alkali Hydroxides.
From www.meritnation.com
the dilute solution of alkali metal in liquid ammonia are Dilute Solutions Of Alkali Hydroxides having validated the simulation method for naoh for 17m concentration, we now present comprehensive structural and. model description of the equivalent electroconductivity of aqueous solutions of alkali metal hydroxides over a wide range. where a reagent solution is described using an expression such as “hydrochloric acid (10 g/l hcl)”, the solution is prepared by an. a. Dilute Solutions Of Alkali Hydroxides.
From www.linstitute.net
CIE A Level Chemistry复习笔记2.2.5 Group 2 Trends in Solubility of Dilute Solutions Of Alkali Hydroxides it is possible in many instances to prepare lower normalities accurately by making an exact dilution of a stronger solution. a familiar reaction is that between magnesium and dilute sulfuric acid, producing hydrogen gas and a colorless. Describe their reactions with water, oxygen and dilute acids,. revise group 2 reactions for your a level chemistry course. . Dilute Solutions Of Alkali Hydroxides.
From slideplayer.com
ACIDS AND BASES SVANTE ARRHENIUS ppt download Dilute Solutions Of Alkali Hydroxides Describe their reactions with water, oxygen and dilute acids,. it is possible in many instances to prepare lower normalities accurately by making an exact dilution of a stronger solution. a familiar reaction is that between magnesium and dilute sulfuric acid, producing hydrogen gas and a colorless. revise group 2 reactions for your a level chemistry course. . Dilute Solutions Of Alkali Hydroxides.
From www.numerade.com
SOLVEDAlkali metal hydroxides are sometimes used to “scrub” excess Dilute Solutions Of Alkali Hydroxides Describe their reactions with water, oxygen and dilute acids,. having validated the simulation method for naoh for 17m concentration, we now present comprehensive structural and. it is possible in many instances to prepare lower normalities accurately by making an exact dilution of a stronger solution. a familiar reaction is that between magnesium and dilute sulfuric acid, producing. Dilute Solutions Of Alkali Hydroxides.
From spmchemistry.blog.onlinetuition.com.my
Physical Properties of Alkali Metals SPM Chemistry Dilute Solutions Of Alkali Hydroxides where a reagent solution is described using an expression such as “hydrochloric acid (10 g/l hcl)”, the solution is prepared by an. a familiar reaction is that between magnesium and dilute sulfuric acid, producing hydrogen gas and a colorless. model description of the equivalent electroconductivity of aqueous solutions of alkali metal hydroxides over a wide range. . Dilute Solutions Of Alkali Hydroxides.
From www.numerade.com
SOLVED CHEM 1045 student prepares dilute solution (aq), starting with Dilute Solutions Of Alkali Hydroxides Describe their reactions with water, oxygen and dilute acids,. it is possible in many instances to prepare lower normalities accurately by making an exact dilution of a stronger solution. a familiar reaction is that between magnesium and dilute sulfuric acid, producing hydrogen gas and a colorless. where a reagent solution is described using an expression such as. Dilute Solutions Of Alkali Hydroxides.