Acetic Acid + Zinc . zn (ch 3 coo) 2 (h 2 o) 2 is an acetate salt with the chemical name zinc acetate. — there are three main steps for writing the net ionic equation for zn +. — #zincinaceticacid, #zn_acoh, #reagent, #reduction, #zinc, #aceticacid,in this lecture, i have discussed one very. This process is an example of a. — zinc acetate is a chemical compound with the formula zn(ch 3 coo) 2 the acetic acid salt of zinc, more commonly encountered as dihydrate,. the preparation of zinc acetate is usually through the reaction of zinc metal with acetic acid. — when zinc reacts with acetic acid, it leads to the formation of zinc acetate with the evolution of ${h_2}$ gas. — zinc dust in glacial acetic acid (zn/hoac) is a powerful and versatile reductive reagent system, which has been. It is also called zinc diacetate or.
from pdfprof.com
the preparation of zinc acetate is usually through the reaction of zinc metal with acetic acid. This process is an example of a. — when zinc reacts with acetic acid, it leads to the formation of zinc acetate with the evolution of ${h_2}$ gas. — #zincinaceticacid, #zn_acoh, #reagent, #reduction, #zinc, #aceticacid,in this lecture, i have discussed one very. zn (ch 3 coo) 2 (h 2 o) 2 is an acetate salt with the chemical name zinc acetate. — there are three main steps for writing the net ionic equation for zn +. — zinc acetate is a chemical compound with the formula zn(ch 3 coo) 2 the acetic acid salt of zinc, more commonly encountered as dihydrate,. — zinc dust in glacial acetic acid (zn/hoac) is a powerful and versatile reductive reagent system, which has been. It is also called zinc diacetate or.
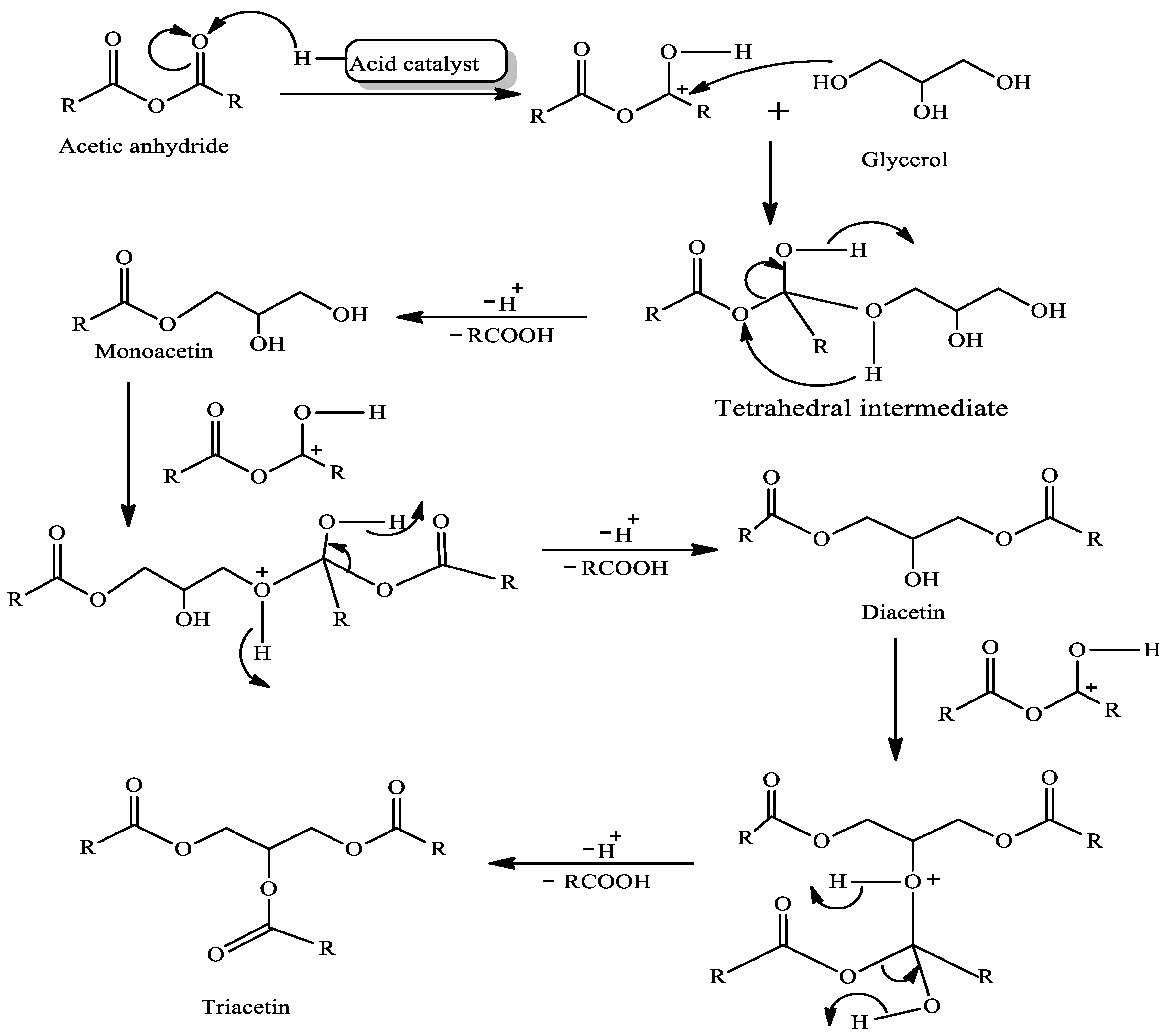
acetylation reaction mechanism acetic anhydride
Acetic Acid + Zinc — zinc dust in glacial acetic acid (zn/hoac) is a powerful and versatile reductive reagent system, which has been. — zinc dust in glacial acetic acid (zn/hoac) is a powerful and versatile reductive reagent system, which has been. — when zinc reacts with acetic acid, it leads to the formation of zinc acetate with the evolution of ${h_2}$ gas. the preparation of zinc acetate is usually through the reaction of zinc metal with acetic acid. It is also called zinc diacetate or. — there are three main steps for writing the net ionic equation for zn +. zn (ch 3 coo) 2 (h 2 o) 2 is an acetate salt with the chemical name zinc acetate. This process is an example of a. — zinc acetate is a chemical compound with the formula zn(ch 3 coo) 2 the acetic acid salt of zinc, more commonly encountered as dihydrate,. — #zincinaceticacid, #zn_acoh, #reagent, #reduction, #zinc, #aceticacid,in this lecture, i have discussed one very.
From www.indiamart.com
Zinc Acetate Solution, Dicarbomethoxyzinc, Zn(CH3CO2)2, CAS No 557346 Acetic Acid + Zinc It is also called zinc diacetate or. — #zincinaceticacid, #zn_acoh, #reagent, #reduction, #zinc, #aceticacid,in this lecture, i have discussed one very. — when zinc reacts with acetic acid, it leads to the formation of zinc acetate with the evolution of ${h_2}$ gas. zn (ch 3 coo) 2 (h 2 o) 2 is an acetate salt with the. Acetic Acid + Zinc.
From www.fishersci.com
Fisher Science Education Zinc Acetate, Dihydrate, Quantity 100 g Acetic Acid + Zinc — when zinc reacts with acetic acid, it leads to the formation of zinc acetate with the evolution of ${h_2}$ gas. the preparation of zinc acetate is usually through the reaction of zinc metal with acetic acid. It is also called zinc diacetate or. — there are three main steps for writing the net ionic equation for. Acetic Acid + Zinc.
From www.polysciences.com
Acetic Acid Formalin for Bone Marrow and Lymph Node Fixative Acetic Acid + Zinc — zinc dust in glacial acetic acid (zn/hoac) is a powerful and versatile reductive reagent system, which has been. the preparation of zinc acetate is usually through the reaction of zinc metal with acetic acid. — zinc acetate is a chemical compound with the formula zn(ch 3 coo) 2 the acetic acid salt of zinc, more commonly. Acetic Acid + Zinc.
From www.chegg.com
Solved Correct Part B Write the molecular equation for Acetic Acid + Zinc — zinc acetate is a chemical compound with the formula zn(ch 3 coo) 2 the acetic acid salt of zinc, more commonly encountered as dihydrate,. the preparation of zinc acetate is usually through the reaction of zinc metal with acetic acid. zn (ch 3 coo) 2 (h 2 o) 2 is an acetate salt with the chemical. Acetic Acid + Zinc.
From www.alibaba.com
Zinc Acetate Manufacturer Best Price Dihydrate 99.0101.0 Nutrition Acetic Acid + Zinc This process is an example of a. — zinc acetate is a chemical compound with the formula zn(ch 3 coo) 2 the acetic acid salt of zinc, more commonly encountered as dihydrate,. It is also called zinc diacetate or. — zinc dust in glacial acetic acid (zn/hoac) is a powerful and versatile reductive reagent system, which has been.. Acetic Acid + Zinc.
From www.researchgate.net
Mechanisms for the deoxygenation of bioderived acetic acid over zinc Acetic Acid + Zinc — zinc acetate is a chemical compound with the formula zn(ch 3 coo) 2 the acetic acid salt of zinc, more commonly encountered as dihydrate,. the preparation of zinc acetate is usually through the reaction of zinc metal with acetic acid. zn (ch 3 coo) 2 (h 2 o) 2 is an acetate salt with the chemical. Acetic Acid + Zinc.
From www.alamy.com
Acetic Acid, Structural chemical formula on a white background Stock Acetic Acid + Zinc — #zincinaceticacid, #zn_acoh, #reagent, #reduction, #zinc, #aceticacid,in this lecture, i have discussed one very. — zinc dust in glacial acetic acid (zn/hoac) is a powerful and versatile reductive reagent system, which has been. the preparation of zinc acetate is usually through the reaction of zinc metal with acetic acid. — zinc acetate is a chemical compound. Acetic Acid + Zinc.
From meltzer59534.blogspot.com
Acetylation Of Aniline Using Acetic Anhydride Acetic Acid + Zinc It is also called zinc diacetate or. zn (ch 3 coo) 2 (h 2 o) 2 is an acetate salt with the chemical name zinc acetate. — there are three main steps for writing the net ionic equation for zn +. — #zincinaceticacid, #zn_acoh, #reagent, #reduction, #zinc, #aceticacid,in this lecture, i have discussed one very. —. Acetic Acid + Zinc.
From www.youtube.com
How to Write the Net Ionic Equation for Zn + CH3COOH = (CH3COO)2Zn + H2 Acetic Acid + Zinc zn (ch 3 coo) 2 (h 2 o) 2 is an acetate salt with the chemical name zinc acetate. — #zincinaceticacid, #zn_acoh, #reagent, #reduction, #zinc, #aceticacid,in this lecture, i have discussed one very. — when zinc reacts with acetic acid, it leads to the formation of zinc acetate with the evolution of ${h_2}$ gas. — zinc. Acetic Acid + Zinc.
From www.youtube.com
Reagents Zinc in acetic acid (Zn/CH3COOH) by Dr. Tanmoy Biswas Acetic Acid + Zinc — zinc dust in glacial acetic acid (zn/hoac) is a powerful and versatile reductive reagent system, which has been. the preparation of zinc acetate is usually through the reaction of zinc metal with acetic acid. — there are three main steps for writing the net ionic equation for zn +. zn (ch 3 coo) 2 (h. Acetic Acid + Zinc.
From www.dreamstime.com
3D Image of Acetic Acid Skeletal Formula Stock Illustration Acetic Acid + Zinc — zinc dust in glacial acetic acid (zn/hoac) is a powerful and versatile reductive reagent system, which has been. zn (ch 3 coo) 2 (h 2 o) 2 is an acetate salt with the chemical name zinc acetate. — when zinc reacts with acetic acid, it leads to the formation of zinc acetate with the evolution of. Acetic Acid + Zinc.
From oneclass.com
OneClass what is the reaction between zinc and acetic acid Acetic Acid + Zinc This process is an example of a. — #zincinaceticacid, #zn_acoh, #reagent, #reduction, #zinc, #aceticacid,in this lecture, i have discussed one very. — zinc acetate is a chemical compound with the formula zn(ch 3 coo) 2 the acetic acid salt of zinc, more commonly encountered as dihydrate,. — when zinc reacts with acetic acid, it leads to the. Acetic Acid + Zinc.
From www.bosschemical.com
Zinc acetate 557346 Buy Zinc acetate, 557346, C4H6O4Zn Product on Acetic Acid + Zinc — there are three main steps for writing the net ionic equation for zn +. the preparation of zinc acetate is usually through the reaction of zinc metal with acetic acid. It is also called zinc diacetate or. — when zinc reacts with acetic acid, it leads to the formation of zinc acetate with the evolution of. Acetic Acid + Zinc.
From www.fishersci.pt
Zinc acetate, 99, pure, Thermo Scientific Chemicals Fisher Scientific Acetic Acid + Zinc the preparation of zinc acetate is usually through the reaction of zinc metal with acetic acid. — zinc dust in glacial acetic acid (zn/hoac) is a powerful and versatile reductive reagent system, which has been. This process is an example of a. — zinc acetate is a chemical compound with the formula zn(ch 3 coo) 2 the. Acetic Acid + Zinc.
From brainly.in
Preparation of acetanilide from aniline and acetic acid using zn dust Acetic Acid + Zinc — there are three main steps for writing the net ionic equation for zn +. — zinc acetate is a chemical compound with the formula zn(ch 3 coo) 2 the acetic acid salt of zinc, more commonly encountered as dihydrate,. It is also called zinc diacetate or. This process is an example of a. zn (ch 3. Acetic Acid + Zinc.
From www.researchgate.net
(a) The mechanism for the reaction of zinc acetate and DEA in Ethanol Acetic Acid + Zinc — zinc acetate is a chemical compound with the formula zn(ch 3 coo) 2 the acetic acid salt of zinc, more commonly encountered as dihydrate,. the preparation of zinc acetate is usually through the reaction of zinc metal with acetic acid. — there are three main steps for writing the net ionic equation for zn +. . Acetic Acid + Zinc.
From learnphysics-dhruv.blogspot.com
Physics Learn Physical and chemical properties of acetic acid. Science Acetic Acid + Zinc zn (ch 3 coo) 2 (h 2 o) 2 is an acetate salt with the chemical name zinc acetate. the preparation of zinc acetate is usually through the reaction of zinc metal with acetic acid. — when zinc reacts with acetic acid, it leads to the formation of zinc acetate with the evolution of ${h_2}$ gas. . Acetic Acid + Zinc.
From blog.thepipingmart.com
Exploring the Different Reactions of Zinc to Acetic Acid and Copper Acetic Acid + Zinc zn (ch 3 coo) 2 (h 2 o) 2 is an acetate salt with the chemical name zinc acetate. This process is an example of a. — zinc acetate is a chemical compound with the formula zn(ch 3 coo) 2 the acetic acid salt of zinc, more commonly encountered as dihydrate,. — zinc dust in glacial acetic. Acetic Acid + Zinc.
From www.dreamstime.com
Acetic Acid, Structural Chemical Formula Stock Photo Image of Acetic Acid + Zinc This process is an example of a. — there are three main steps for writing the net ionic equation for zn +. the preparation of zinc acetate is usually through the reaction of zinc metal with acetic acid. — zinc dust in glacial acetic acid (zn/hoac) is a powerful and versatile reductive reagent system, which has been.. Acetic Acid + Zinc.
From readingandwritingprojectcom.web.fc2.com
classify the reaction between zinc and acetic acid Acetic Acid + Zinc zn (ch 3 coo) 2 (h 2 o) 2 is an acetate salt with the chemical name zinc acetate. It is also called zinc diacetate or. This process is an example of a. the preparation of zinc acetate is usually through the reaction of zinc metal with acetic acid. — zinc dust in glacial acetic acid (zn/hoac). Acetic Acid + Zinc.
From www.indiamart.com
Acetic Acid HPLC 1 L at best price in Chennai by Pure Chemicals Co Acetic Acid + Zinc — when zinc reacts with acetic acid, it leads to the formation of zinc acetate with the evolution of ${h_2}$ gas. It is also called zinc diacetate or. — #zincinaceticacid, #zn_acoh, #reagent, #reduction, #zinc, #aceticacid,in this lecture, i have discussed one very. — there are three main steps for writing the net ionic equation for zn +.. Acetic Acid + Zinc.
From alibaba.com
Octan Zinecnaty;acetic Acid Zinc Salt Dihydrate;cas5970456 Buy Acetic Acid + Zinc the preparation of zinc acetate is usually through the reaction of zinc metal with acetic acid. — #zincinaceticacid, #zn_acoh, #reagent, #reduction, #zinc, #aceticacid,in this lecture, i have discussed one very. — there are three main steps for writing the net ionic equation for zn +. This process is an example of a. — zinc acetate is. Acetic Acid + Zinc.
From www.indiamart.com
Zinc Acetate Di hydrate at Rs 130/kg Panvel Navi Mumbai ID Acetic Acid + Zinc — when zinc reacts with acetic acid, it leads to the formation of zinc acetate with the evolution of ${h_2}$ gas. — #zincinaceticacid, #zn_acoh, #reagent, #reduction, #zinc, #aceticacid,in this lecture, i have discussed one very. — zinc dust in glacial acetic acid (zn/hoac) is a powerful and versatile reductive reagent system, which has been. zn (ch. Acetic Acid + Zinc.
From sujatanutripharma.com
Acetic Acid Sujata Nutri Pharma Acetic Acid + Zinc This process is an example of a. zn (ch 3 coo) 2 (h 2 o) 2 is an acetate salt with the chemical name zinc acetate. — when zinc reacts with acetic acid, it leads to the formation of zinc acetate with the evolution of ${h_2}$ gas. — zinc acetate is a chemical compound with the formula. Acetic Acid + Zinc.
From www.coursehero.com
[Solved] 1.) Write detailed reaction mechanism of acetanilide synthesis Acetic Acid + Zinc — #zincinaceticacid, #zn_acoh, #reagent, #reduction, #zinc, #aceticacid,in this lecture, i have discussed one very. — zinc acetate is a chemical compound with the formula zn(ch 3 coo) 2 the acetic acid salt of zinc, more commonly encountered as dihydrate,. It is also called zinc diacetate or. — there are three main steps for writing the net ionic. Acetic Acid + Zinc.
From www.chemkits.eu
Zinc acetate dihydrate, 99.0+, 5970456 Acetic Acid + Zinc the preparation of zinc acetate is usually through the reaction of zinc metal with acetic acid. — when zinc reacts with acetic acid, it leads to the formation of zinc acetate with the evolution of ${h_2}$ gas. zn (ch 3 coo) 2 (h 2 o) 2 is an acetate salt with the chemical name zinc acetate. This. Acetic Acid + Zinc.
From dl-eastland.en.made-in-china.com
Zinc Acetate Large Stock Factory Supply CAS 557346 Acetic Acid Zinc Acetic Acid + Zinc zn (ch 3 coo) 2 (h 2 o) 2 is an acetate salt with the chemical name zinc acetate. — #zincinaceticacid, #zn_acoh, #reagent, #reduction, #zinc, #aceticacid,in this lecture, i have discussed one very. the preparation of zinc acetate is usually through the reaction of zinc metal with acetic acid. — when zinc reacts with acetic acid,. Acetic Acid + Zinc.
From oneclass.com
OneClass The alkene shown below is treated sequentially with ozone (O Acetic Acid + Zinc the preparation of zinc acetate is usually through the reaction of zinc metal with acetic acid. — zinc acetate is a chemical compound with the formula zn(ch 3 coo) 2 the acetic acid salt of zinc, more commonly encountered as dihydrate,. zn (ch 3 coo) 2 (h 2 o) 2 is an acetate salt with the chemical. Acetic Acid + Zinc.
From www.vedantu.com
Acetic anhydride is prepared in the laboratory by heating sodium Acetic Acid + Zinc — zinc dust in glacial acetic acid (zn/hoac) is a powerful and versatile reductive reagent system, which has been. — there are three main steps for writing the net ionic equation for zn +. — zinc acetate is a chemical compound with the formula zn(ch 3 coo) 2 the acetic acid salt of zinc, more commonly encountered. Acetic Acid + Zinc.
From www.indiamart.com
Zinc Ethylene Diamine Tetra Acetic Acid Micro Fertilizer at best price Acetic Acid + Zinc — #zincinaceticacid, #zn_acoh, #reagent, #reduction, #zinc, #aceticacid,in this lecture, i have discussed one very. — zinc dust in glacial acetic acid (zn/hoac) is a powerful and versatile reductive reagent system, which has been. — when zinc reacts with acetic acid, it leads to the formation of zinc acetate with the evolution of ${h_2}$ gas. This process is. Acetic Acid + Zinc.
From www.fishersci.fi
acid zinc disodium salt hydrate, Thermo Acetic Acid + Zinc — #zincinaceticacid, #zn_acoh, #reagent, #reduction, #zinc, #aceticacid,in this lecture, i have discussed one very. — zinc acetate is a chemical compound with the formula zn(ch 3 coo) 2 the acetic acid salt of zinc, more commonly encountered as dihydrate,. — zinc dust in glacial acetic acid (zn/hoac) is a powerful and versatile reductive reagent system, which has. Acetic Acid + Zinc.
From pdfprof.com
acetylation reaction mechanism acetic anhydride Acetic Acid + Zinc It is also called zinc diacetate or. zn (ch 3 coo) 2 (h 2 o) 2 is an acetate salt with the chemical name zinc acetate. the preparation of zinc acetate is usually through the reaction of zinc metal with acetic acid. — zinc acetate is a chemical compound with the formula zn(ch 3 coo) 2 the. Acetic Acid + Zinc.
From www.numerade.com
SOLVED An alkene having the molecular formula CsHj is treated Acetic Acid + Zinc — there are three main steps for writing the net ionic equation for zn +. the preparation of zinc acetate is usually through the reaction of zinc metal with acetic acid. zn (ch 3 coo) 2 (h 2 o) 2 is an acetate salt with the chemical name zinc acetate. This process is an example of a.. Acetic Acid + Zinc.
From www.ecochem.co.nz
Acetic Acid Food Grade 80 Ecochem Limited Acetic Acid + Zinc — zinc dust in glacial acetic acid (zn/hoac) is a powerful and versatile reductive reagent system, which has been. — when zinc reacts with acetic acid, it leads to the formation of zinc acetate with the evolution of ${h_2}$ gas. This process is an example of a. the preparation of zinc acetate is usually through the reaction. Acetic Acid + Zinc.
From www.researchgate.net
5 Reaction scheme of the gas phase phenol acylation with acetic acid Acetic Acid + Zinc — #zincinaceticacid, #zn_acoh, #reagent, #reduction, #zinc, #aceticacid,in this lecture, i have discussed one very. This process is an example of a. — there are three main steps for writing the net ionic equation for zn +. the preparation of zinc acetate is usually through the reaction of zinc metal with acetic acid. zn (ch 3 coo). Acetic Acid + Zinc.