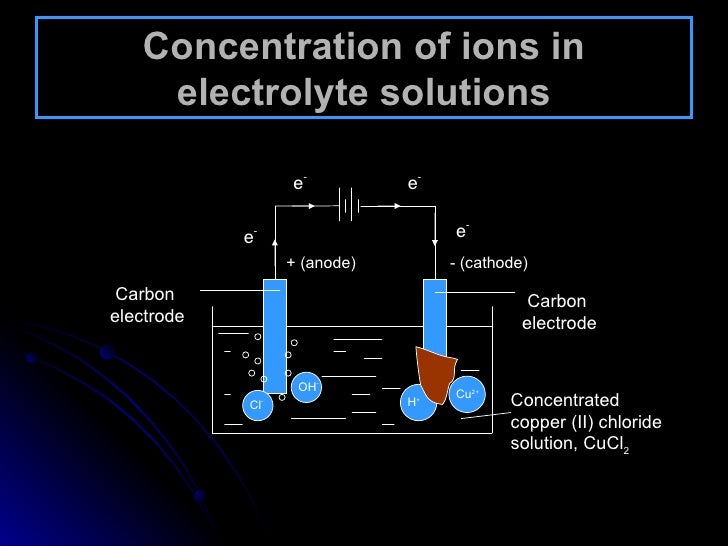
Electrolyte Concentration Cell Example . Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent. A concentration cell is an electrochemical cell where both electrodes are made of the same material, but they are. The electrolyte concentration cell mainly consists of identical electrodes which are immersed in the solutions of the same electrolytes. A voltage can also be generated by constructing an electrochemical cell in which each compartment contains the same redox active solution but at different. Concentration cells are composed of the same electrode and electrolyte solution with different concentrations which gives the cell potential. These cells consist of identical electrodes immersed in the solutions of the same electrolytes but with varying concentrations. In these cells, the two electrodes of the same metal are dipping in solutions of metal ions of different.
from www.slideshare.net
These cells consist of identical electrodes immersed in the solutions of the same electrolytes but with varying concentrations. A voltage can also be generated by constructing an electrochemical cell in which each compartment contains the same redox active solution but at different. A concentration cell is an electrochemical cell where both electrodes are made of the same material, but they are. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent. In these cells, the two electrodes of the same metal are dipping in solutions of metal ions of different. Concentration cells are composed of the same electrode and electrolyte solution with different concentrations which gives the cell potential. The electrolyte concentration cell mainly consists of identical electrodes which are immersed in the solutions of the same electrolytes.
Chapter 6 electrochemistry
Electrolyte Concentration Cell Example A concentration cell is an electrochemical cell where both electrodes are made of the same material, but they are. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent. These cells consist of identical electrodes immersed in the solutions of the same electrolytes but with varying concentrations. In these cells, the two electrodes of the same metal are dipping in solutions of metal ions of different. A voltage can also be generated by constructing an electrochemical cell in which each compartment contains the same redox active solution but at different. Concentration cells are composed of the same electrode and electrolyte solution with different concentrations which gives the cell potential. The electrolyte concentration cell mainly consists of identical electrodes which are immersed in the solutions of the same electrolytes. A concentration cell is an electrochemical cell where both electrodes are made of the same material, but they are.
From courses.lumenlearning.com
Electrolytes Chemistry for Majors Electrolyte Concentration Cell Example In these cells, the two electrodes of the same metal are dipping in solutions of metal ions of different. The electrolyte concentration cell mainly consists of identical electrodes which are immersed in the solutions of the same electrolytes. These cells consist of identical electrodes immersed in the solutions of the same electrolytes but with varying concentrations. Electrolytic cells are very. Electrolyte Concentration Cell Example.
From www.youtube.com
ELECTROCHEMISTRY ELECTROLYTE CONCENTRATION CELL WITHOUT TRANSFERENCE Electrolyte Concentration Cell Example In these cells, the two electrodes of the same metal are dipping in solutions of metal ions of different. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent. The electrolyte concentration cell mainly consists of identical electrodes which are. Electrolyte Concentration Cell Example.
From www.slideserve.com
PPT 26 PowerPoint Presentation, free download ID3223810 Electrolyte Concentration Cell Example These cells consist of identical electrodes immersed in the solutions of the same electrolytes but with varying concentrations. The electrolyte concentration cell mainly consists of identical electrodes which are immersed in the solutions of the same electrolytes. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and. Electrolyte Concentration Cell Example.
From www.slideshare.net
Fluids & Electrolyte Electrolyte Concentration Cell Example These cells consist of identical electrodes immersed in the solutions of the same electrolytes but with varying concentrations. Concentration cells are composed of the same electrode and electrolyte solution with different concentrations which gives the cell potential. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and. Electrolyte Concentration Cell Example.
From slideplayer.com
ERT 108 PHYSICAL CHEMISTRY Equilibrium Electrochemistry By; Mrs Haf Electrolyte Concentration Cell Example The electrolyte concentration cell mainly consists of identical electrodes which are immersed in the solutions of the same electrolytes. Concentration cells are composed of the same electrode and electrolyte solution with different concentrations which gives the cell potential. A concentration cell is an electrochemical cell where both electrodes are made of the same material, but they are. Electrolytic cells are. Electrolyte Concentration Cell Example.
From www.slideshare.net
Fluids & Electrolyte Electrolyte Concentration Cell Example The electrolyte concentration cell mainly consists of identical electrodes which are immersed in the solutions of the same electrolytes. A concentration cell is an electrochemical cell where both electrodes are made of the same material, but they are. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode. Electrolyte Concentration Cell Example.
From www.slideserve.com
PPT Lecture 13 The Nernst Equation PowerPoint Presentation, free Electrolyte Concentration Cell Example Concentration cells are composed of the same electrode and electrolyte solution with different concentrations which gives the cell potential. A concentration cell is an electrochemical cell where both electrodes are made of the same material, but they are. These cells consist of identical electrodes immersed in the solutions of the same electrolytes but with varying concentrations. In these cells, the. Electrolyte Concentration Cell Example.
From www.youtube.com
EMF of Electrolyte Concentration Cell Reversible to Cation with Electrolyte Concentration Cell Example A concentration cell is an electrochemical cell where both electrodes are made of the same material, but they are. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent. These cells consist of identical electrodes immersed in the solutions of. Electrolyte Concentration Cell Example.
From www.youtube.com
Electrolyte Concentration cell With Transference (Reversible to Anion Electrolyte Concentration Cell Example Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent. In these cells, the two electrodes of the same metal are dipping in solutions of metal ions of different. Concentration cells are composed of the same electrode and electrolyte solution. Electrolyte Concentration Cell Example.
From www.youtube.com
Electrochemistry The Concentration Cell. YouTube Electrolyte Concentration Cell Example A voltage can also be generated by constructing an electrochemical cell in which each compartment contains the same redox active solution but at different. Concentration cells are composed of the same electrode and electrolyte solution with different concentrations which gives the cell potential. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt. Electrolyte Concentration Cell Example.
From www.youtube.com
Electrochemistry Lecture 5 (Electrode & Electrolyte Concentration Electrolyte Concentration Cell Example Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent. A concentration cell is an electrochemical cell where both electrodes are made of the same material, but they are. The electrolyte concentration cell mainly consists of identical electrodes which are. Electrolyte Concentration Cell Example.
From www.slideserve.com
PPT Body Major Electrolytes PowerPoint Presentation, free download Electrolyte Concentration Cell Example In these cells, the two electrodes of the same metal are dipping in solutions of metal ions of different. A voltage can also be generated by constructing an electrochemical cell in which each compartment contains the same redox active solution but at different. The electrolyte concentration cell mainly consists of identical electrodes which are immersed in the solutions of the. Electrolyte Concentration Cell Example.
From chem.libretexts.org
Electrolytic Cells Chemistry LibreTexts Electrolyte Concentration Cell Example Concentration cells are composed of the same electrode and electrolyte solution with different concentrations which gives the cell potential. A voltage can also be generated by constructing an electrochemical cell in which each compartment contains the same redox active solution but at different. A concentration cell is an electrochemical cell where both electrodes are made of the same material, but. Electrolyte Concentration Cell Example.
From www.youtube.com
Electrochemistry Lecture 7 (Electrolyte Concentration Cells With Electrolyte Concentration Cell Example A concentration cell is an electrochemical cell where both electrodes are made of the same material, but they are. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent. In these cells, the two electrodes of the same metal are. Electrolyte Concentration Cell Example.
From www.youtube.com
Electrolyte Concentration cell Electrochemistry 2 YouTube Electrolyte Concentration Cell Example Concentration cells are composed of the same electrode and electrolyte solution with different concentrations which gives the cell potential. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent. A voltage can also be generated by constructing an electrochemical cell. Electrolyte Concentration Cell Example.
From www.scribd.com
Concentration Cells Redox Electrolyte Electrolyte Concentration Cell Example In these cells, the two electrodes of the same metal are dipping in solutions of metal ions of different. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent. Concentration cells are composed of the same electrode and electrolyte solution. Electrolyte Concentration Cell Example.
From www.youtube.com
C.6 Concentration cells (HL) YouTube Electrolyte Concentration Cell Example The electrolyte concentration cell mainly consists of identical electrodes which are immersed in the solutions of the same electrolytes. Concentration cells are composed of the same electrode and electrolyte solution with different concentrations which gives the cell potential. A voltage can also be generated by constructing an electrochemical cell in which each compartment contains the same redox active solution but. Electrolyte Concentration Cell Example.
From sciencevision.in
Electrolytes , Electolytic Cell And Electrochemical Cell Science Vision Electrolyte Concentration Cell Example The electrolyte concentration cell mainly consists of identical electrodes which are immersed in the solutions of the same electrolytes. A concentration cell is an electrochemical cell where both electrodes are made of the same material, but they are. A voltage can also be generated by constructing an electrochemical cell in which each compartment contains the same redox active solution but. Electrolyte Concentration Cell Example.
From slideplayer.com
Electrochemical Cell. ppt download Electrolyte Concentration Cell Example These cells consist of identical electrodes immersed in the solutions of the same electrolytes but with varying concentrations. A concentration cell is an electrochemical cell where both electrodes are made of the same material, but they are. Concentration cells are composed of the same electrode and electrolyte solution with different concentrations which gives the cell potential. The electrolyte concentration cell. Electrolyte Concentration Cell Example.
From www.straighthealthcare.com
Electrolytes Electrolyte Concentration Cell Example Concentration cells are composed of the same electrode and electrolyte solution with different concentrations which gives the cell potential. A concentration cell is an electrochemical cell where both electrodes are made of the same material, but they are. The electrolyte concentration cell mainly consists of identical electrodes which are immersed in the solutions of the same electrolytes. A voltage can. Electrolyte Concentration Cell Example.
From www.youtube.com
Electrolyte Concentration Cell Without Transference Solved Examples Electrolyte Concentration Cell Example Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent. A voltage can also be generated by constructing an electrochemical cell in which each compartment contains the same redox active solution but at different. These cells consist of identical electrodes. Electrolyte Concentration Cell Example.
From www.slideserve.com
PPT Chemistry 232 PowerPoint Presentation, free download ID2317752 Electrolyte Concentration Cell Example A voltage can also be generated by constructing an electrochemical cell in which each compartment contains the same redox active solution but at different. A concentration cell is an electrochemical cell where both electrodes are made of the same material, but they are. These cells consist of identical electrodes immersed in the solutions of the same electrolytes but with varying. Electrolyte Concentration Cell Example.
From www.youtube.com
Electrochemistry Lecture 6 (Electrolyte Concentration Cells Without Electrolyte Concentration Cell Example A concentration cell is an electrochemical cell where both electrodes are made of the same material, but they are. In these cells, the two electrodes of the same metal are dipping in solutions of metal ions of different. The electrolyte concentration cell mainly consists of identical electrodes which are immersed in the solutions of the same electrolytes. These cells consist. Electrolyte Concentration Cell Example.
From jackwestin.com
Concentration Cell Electrochemistry MCAT Content Electrolyte Concentration Cell Example A voltage can also be generated by constructing an electrochemical cell in which each compartment contains the same redox active solution but at different. Concentration cells are composed of the same electrode and electrolyte solution with different concentrations which gives the cell potential. In these cells, the two electrodes of the same metal are dipping in solutions of metal ions. Electrolyte Concentration Cell Example.
From webmis.highland.cc.il.us
Electrolysis Electrolyte Concentration Cell Example Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent. These cells consist of identical electrodes immersed in the solutions of the same electrolytes but with varying concentrations. Concentration cells are composed of the same electrode and electrolyte solution with. Electrolyte Concentration Cell Example.
From www.slideserve.com
PPT Lecture 13 The Nernst Equation PowerPoint Presentation, free Electrolyte Concentration Cell Example A concentration cell is an electrochemical cell where both electrodes are made of the same material, but they are. In these cells, the two electrodes of the same metal are dipping in solutions of metal ions of different. Concentration cells are composed of the same electrode and electrolyte solution with different concentrations which gives the cell potential. These cells consist. Electrolyte Concentration Cell Example.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2281241 Electrolyte Concentration Cell Example In these cells, the two electrodes of the same metal are dipping in solutions of metal ions of different. A voltage can also be generated by constructing an electrochemical cell in which each compartment contains the same redox active solution but at different. A concentration cell is an electrochemical cell where both electrodes are made of the same material, but. Electrolyte Concentration Cell Example.
From slidetodoc.com
Lecture 9 Electrical conductivity of electrolytes solutions Prepared Electrolyte Concentration Cell Example The electrolyte concentration cell mainly consists of identical electrodes which are immersed in the solutions of the same electrolytes. In these cells, the two electrodes of the same metal are dipping in solutions of metal ions of different. A voltage can also be generated by constructing an electrochemical cell in which each compartment contains the same redox active solution but. Electrolyte Concentration Cell Example.
From flatworldknowledge.lardbucket.org
Nutrients Important to Fluid and Electrolyte Balance Electrolyte Concentration Cell Example A voltage can also be generated by constructing an electrochemical cell in which each compartment contains the same redox active solution but at different. In these cells, the two electrodes of the same metal are dipping in solutions of metal ions of different. A concentration cell is an electrochemical cell where both electrodes are made of the same material, but. Electrolyte Concentration Cell Example.
From www.researchgate.net
Cell schematic with an electrolyte of varying concentration equipped Electrolyte Concentration Cell Example These cells consist of identical electrodes immersed in the solutions of the same electrolytes but with varying concentrations. A concentration cell is an electrochemical cell where both electrodes are made of the same material, but they are. The electrolyte concentration cell mainly consists of identical electrodes which are immersed in the solutions of the same electrolytes. Electrolytic cells are very. Electrolyte Concentration Cell Example.
From www.slideserve.com
PPT Fluids and Electrolytes Balance PowerPoint Presentation, free Electrolyte Concentration Cell Example Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent. These cells consist of identical electrodes immersed in the solutions of the same electrolytes but with varying concentrations. Concentration cells are composed of the same electrode and electrolyte solution with. Electrolyte Concentration Cell Example.
From www.chegg.com
Solved 1. Sketch a typical Electrolyte Concentration cell Electrolyte Concentration Cell Example A concentration cell is an electrochemical cell where both electrodes are made of the same material, but they are. The electrolyte concentration cell mainly consists of identical electrodes which are immersed in the solutions of the same electrolytes. Concentration cells are composed of the same electrode and electrolyte solution with different concentrations which gives the cell potential. In these cells,. Electrolyte Concentration Cell Example.
From www.youtube.com
EMF of Electrolyte Concentration Cell Reversible to Cations without Electrolyte Concentration Cell Example A voltage can also be generated by constructing an electrochemical cell in which each compartment contains the same redox active solution but at different. The electrolyte concentration cell mainly consists of identical electrodes which are immersed in the solutions of the same electrolytes. In these cells, the two electrodes of the same metal are dipping in solutions of metal ions. Electrolyte Concentration Cell Example.
From schoolbag.info
Figure 12.3. The Cell Membrane as an Example of a Concentration Cell Electrolyte Concentration Cell Example A concentration cell is an electrochemical cell where both electrodes are made of the same material, but they are. Concentration cells are composed of the same electrode and electrolyte solution with different concentrations which gives the cell potential. The electrolyte concentration cell mainly consists of identical electrodes which are immersed in the solutions of the same electrolytes. These cells consist. Electrolyte Concentration Cell Example.
From www.slideshare.net
Chapter 6 electrochemistry Electrolyte Concentration Cell Example A voltage can also be generated by constructing an electrochemical cell in which each compartment contains the same redox active solution but at different. The electrolyte concentration cell mainly consists of identical electrodes which are immersed in the solutions of the same electrolytes. In these cells, the two electrodes of the same metal are dipping in solutions of metal ions. Electrolyte Concentration Cell Example.