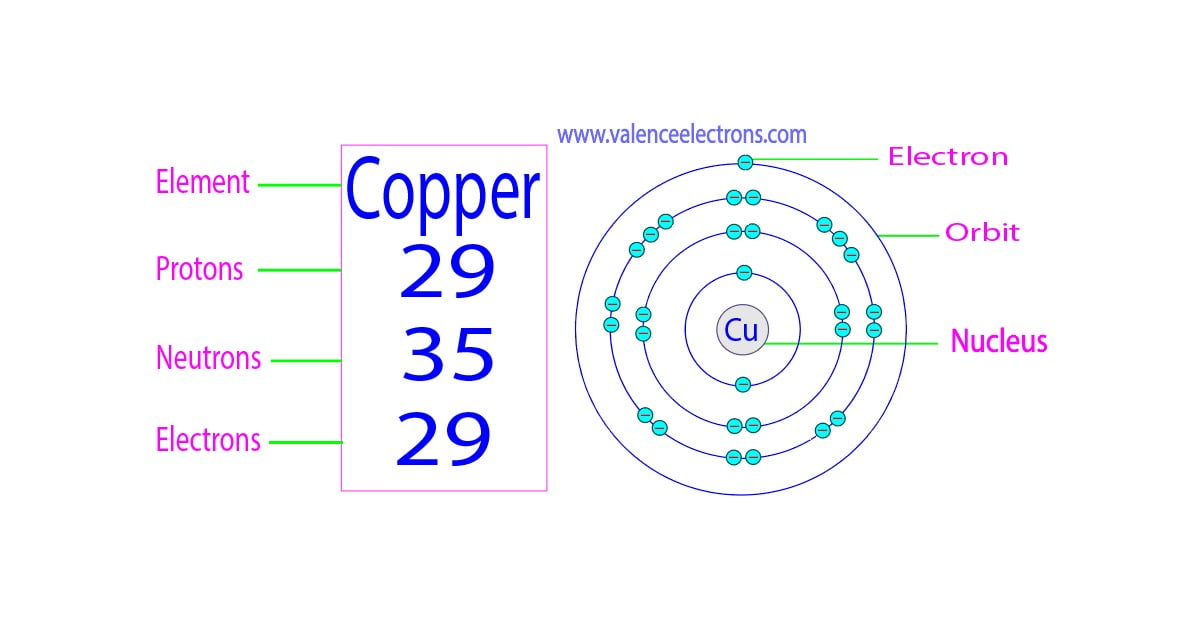
Copper Electrons Per Shell . how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. the electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. The first shell has 2 electrons, the second shell has. copper is a chemical element of the periodic table with chemical symbol cu and atomic number 29 with an atomic weight of. copper has 29 electrons distributed in its electron shells. copper atoms have 29 electrons and the shell structure is 2.8.18.1. The ground state electron configuration of. There is a formula for obtaining the. the third shell can carry up 18 electrons, but it is more stable by carrying only eight electrons.
from elchoroukhost.net
the third shell can carry up 18 electrons, but it is more stable by carrying only eight electrons. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. copper atoms have 29 electrons and the shell structure is 2.8.18.1. copper has 29 electrons distributed in its electron shells. There is a formula for obtaining the. The ground state electron configuration of. the electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. The first shell has 2 electrons, the second shell has. copper is a chemical element of the periodic table with chemical symbol cu and atomic number 29 with an atomic weight of.
Copper Periodic Table Protons Neutrons And Electrons Elcho Table
Copper Electrons Per Shell copper atoms have 29 electrons and the shell structure is 2.8.18.1. copper atoms have 29 electrons and the shell structure is 2.8.18.1. The ground state electron configuration of. the electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. There is a formula for obtaining the. copper is a chemical element of the periodic table with chemical symbol cu and atomic number 29 with an atomic weight of. The first shell has 2 electrons, the second shell has. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. the third shell can carry up 18 electrons, but it is more stable by carrying only eight electrons. copper has 29 electrons distributed in its electron shells.
From www.slideserve.com
PPT How are electrons arranged? PowerPoint Presentation, free Copper Electrons Per Shell how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. the electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. The ground state electron configuration of. The first shell has 2 electrons,. Copper Electrons Per Shell.
From chemistrytalk.org
Electron Shells ChemTalk Copper Electrons Per Shell There is a formula for obtaining the. copper atoms have 29 electrons and the shell structure is 2.8.18.1. The ground state electron configuration of. The first shell has 2 electrons, the second shell has. the third shell can carry up 18 electrons, but it is more stable by carrying only eight electrons. the electron distribution in copper. Copper Electrons Per Shell.
From www.alamy.com
Colorful Periodic Table of the Elements shows atomic number, symbol Copper Electrons Per Shell copper atoms have 29 electrons and the shell structure is 2.8.18.1. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. There is a formula for obtaining the. The first shell has 2 electrons, the second shell has. copper is a chemical element of the. Copper Electrons Per Shell.
From www.slideshare.net
Copper Copper Electrons Per Shell copper is a chemical element of the periodic table with chemical symbol cu and atomic number 29 with an atomic weight of. the electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. the third shell can carry up 18 electrons, but it is. Copper Electrons Per Shell.
From www.britannica.com
Electron shell Definition & Facts Britannica Copper Electrons Per Shell the third shell can carry up 18 electrons, but it is more stable by carrying only eight electrons. The first shell has 2 electrons, the second shell has. There is a formula for obtaining the. copper atoms have 29 electrons and the shell structure is 2.8.18.1. the electron distribution in copper spans across the k, l, m,. Copper Electrons Per Shell.
From ar.inspiredpencil.com
Copper Atomic Structure Copper Electrons Per Shell There is a formula for obtaining the. copper atoms have 29 electrons and the shell structure is 2.8.18.1. copper is a chemical element of the periodic table with chemical symbol cu and atomic number 29 with an atomic weight of. copper has 29 electrons distributed in its electron shells. The first shell has 2 electrons, the second. Copper Electrons Per Shell.
From material-properties.org
Copper Periodic Table and Atomic Properties Copper Electrons Per Shell There is a formula for obtaining the. The first shell has 2 electrons, the second shell has. copper is a chemical element of the periodic table with chemical symbol cu and atomic number 29 with an atomic weight of. the third shell can carry up 18 electrons, but it is more stable by carrying only eight electrons. . Copper Electrons Per Shell.
From www.alamy.com
Copper atom hires stock photography and images Alamy Copper Electrons Per Shell copper atoms have 29 electrons and the shell structure is 2.8.18.1. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. There is a formula for obtaining the. the electron distribution in copper spans across the k, l, m, and n shells, with valence electrons. Copper Electrons Per Shell.
From en-academic.com
Copper Copper Electrons Per Shell copper has 29 electrons distributed in its electron shells. The ground state electron configuration of. The first shell has 2 electrons, the second shell has. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. copper is a chemical element of the periodic table with. Copper Electrons Per Shell.
From www.dreamstime.com
Electron of the Element Copper Stock Vector Illustration of Copper Electrons Per Shell The ground state electron configuration of. the third shell can carry up 18 electrons, but it is more stable by carrying only eight electrons. There is a formula for obtaining the. copper is a chemical element of the periodic table with chemical symbol cu and atomic number 29 with an atomic weight of. copper atoms have 29. Copper Electrons Per Shell.
From www.alamy.com
Copper (Cu). Diagram of the nuclear composition, electron configuration Copper Electrons Per Shell There is a formula for obtaining the. The ground state electron configuration of. copper has 29 electrons distributed in its electron shells. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. copper atoms have 29 electrons and the shell structure is 2.8.18.1. copper. Copper Electrons Per Shell.
From izayahmeowcrosby.blogspot.com
Electronic Configuration of Copper Copper Electrons Per Shell the electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. copper has 29 electrons distributed in its electron shells. the third shell can carry up 18 electrons, but it is more stable by carrying only eight electrons. The ground state electron configuration of.. Copper Electrons Per Shell.
From sciencenotes.org
Electron Shell Diagrams of the 118 Elements Copper Electrons Per Shell The ground state electron configuration of. There is a formula for obtaining the. the third shell can carry up 18 electrons, but it is more stable by carrying only eight electrons. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. copper has 29 electrons. Copper Electrons Per Shell.
From www.youtube.com
How to Find the Valence Electrons for Copper (Cu) YouTube Copper Electrons Per Shell copper atoms have 29 electrons and the shell structure is 2.8.18.1. The ground state electron configuration of. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. copper has 29 electrons distributed in its electron shells. There is a formula for obtaining the. copper. Copper Electrons Per Shell.
From www.alamy.com
Periodic Table of the Elements shows atomic number, symbol, name Copper Electrons Per Shell There is a formula for obtaining the. the electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. The ground state electron configuration of. copper atoms have 29 electrons and the shell structure is 2.8.18.1. copper has 29 electrons distributed in its electron shells.. Copper Electrons Per Shell.
From www.earthdate.org
Copper’s Superpower EarthDate Copper Electrons Per Shell copper atoms have 29 electrons and the shell structure is 2.8.18.1. the third shell can carry up 18 electrons, but it is more stable by carrying only eight electrons. the electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. There is a formula. Copper Electrons Per Shell.
From www.sciencephoto.com
Copper, atomic structure Stock Image C018/3710 Science Photo Library Copper Electrons Per Shell There is a formula for obtaining the. copper has 29 electrons distributed in its electron shells. copper atoms have 29 electrons and the shell structure is 2.8.18.1. the electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. The ground state electron configuration of.. Copper Electrons Per Shell.
From www.mooramo.com
Ions of Transition Elements Mooramo Copper Electrons Per Shell copper is a chemical element of the periodic table with chemical symbol cu and atomic number 29 with an atomic weight of. copper atoms have 29 electrons and the shell structure is 2.8.18.1. copper has 29 electrons distributed in its electron shells. The first shell has 2 electrons, the second shell has. There is a formula for. Copper Electrons Per Shell.
From stock.adobe.com
Cu Copper Element Information Facts, Properties, Trends, Uses and Copper Electrons Per Shell the third shell can carry up 18 electrons, but it is more stable by carrying only eight electrons. copper has 29 electrons distributed in its electron shells. copper atoms have 29 electrons and the shell structure is 2.8.18.1. copper is a chemical element of the periodic table with chemical symbol cu and atomic number 29 with. Copper Electrons Per Shell.
From www.youtube.com
Copper Electron Configuration Organic Chemistry Examples YouTube Copper Electrons Per Shell how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. There is a formula for obtaining the. copper atoms have 29 electrons and the shell structure is 2.8.18.1. the electron distribution in copper spans across the k, l, m, and n shells, with valence electrons. Copper Electrons Per Shell.
From www.alamy.com
3d render of atom structure of copper isolated over white background Copper Electrons Per Shell There is a formula for obtaining the. The ground state electron configuration of. copper is a chemical element of the periodic table with chemical symbol cu and atomic number 29 with an atomic weight of. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. . Copper Electrons Per Shell.
From elchoroukhost.net
Copper Periodic Table Protons Neutrons And Electrons Elcho Table Copper Electrons Per Shell copper atoms have 29 electrons and the shell structure is 2.8.18.1. The first shell has 2 electrons, the second shell has. The ground state electron configuration of. There is a formula for obtaining the. the electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals.. Copper Electrons Per Shell.
From www.alamy.com
Copper (Cu). Diagram of the nuclear composition and electron Copper Electrons Per Shell copper has 29 electrons distributed in its electron shells. the third shell can carry up 18 electrons, but it is more stable by carrying only eight electrons. copper atoms have 29 electrons and the shell structure is 2.8.18.1. copper is a chemical element of the periodic table with chemical symbol cu and atomic number 29 with. Copper Electrons Per Shell.
From www.vectorstock.com
Symbol and electron diagram for copper Royalty Free Vector Copper Electrons Per Shell the electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. copper atoms have 29 electrons and the shell structure is 2.8.18.1. copper has 29 electrons distributed in its electron shells. copper is a chemical element of the periodic table with chemical symbol. Copper Electrons Per Shell.
From valenceelectrons.com
How Many Valence Electrons Does Copper (Cu) Have? Copper Electrons Per Shell how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. The first shell has 2 electrons, the second shell has. the electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. copper. Copper Electrons Per Shell.
From www.seekpng.com
Electron Shell 020 Calcium Copper Electron Shell Diagram PNG Image Copper Electrons Per Shell There is a formula for obtaining the. The first shell has 2 electrons, the second shell has. the electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. copper is a chemical element of the periodic table with chemical symbol cu and atomic number 29. Copper Electrons Per Shell.
From www.alamy.com
Periodic Table of the Elements shows atomic number, symbol, name Copper Electrons Per Shell copper atoms have 29 electrons and the shell structure is 2.8.18.1. copper is a chemical element of the periodic table with chemical symbol cu and atomic number 29 with an atomic weight of. The first shell has 2 electrons, the second shell has. the third shell can carry up 18 electrons, but it is more stable by. Copper Electrons Per Shell.
From www.sciencefacts.net
Electron Shell Definition & Number of Electrons in Each Shell Copper Electrons Per Shell copper has 29 electrons distributed in its electron shells. The ground state electron configuration of. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. The first shell has 2 electrons, the second shell has. the third shell can carry up 18 electrons, but it. Copper Electrons Per Shell.
From valenceelectrons.com
Electron Configuration for Copper (Cu, Cu+, Cu2+) Copper Electrons Per Shell copper has 29 electrons distributed in its electron shells. the third shell can carry up 18 electrons, but it is more stable by carrying only eight electrons. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. There is a formula for obtaining the. . Copper Electrons Per Shell.
From general.chemistrysteps.com
Orbital Diagrams Chemistry Steps Copper Electrons Per Shell The first shell has 2 electrons, the second shell has. copper atoms have 29 electrons and the shell structure is 2.8.18.1. the electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. copper has 29 electrons distributed in its electron shells. how to. Copper Electrons Per Shell.
From www.alamy.com
Colorful Periodic Table of the Elements shows atomic number, symbol Copper Electrons Per Shell copper is a chemical element of the periodic table with chemical symbol cu and atomic number 29 with an atomic weight of. copper atoms have 29 electrons and the shell structure is 2.8.18.1. There is a formula for obtaining the. The ground state electron configuration of. how to write the electron configuration for copper (cu, cu+, and. Copper Electrons Per Shell.
From sciencenotes.org
Periodic Table Showing Shells Copper Electrons Per Shell copper has 29 electrons distributed in its electron shells. the electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. copper atoms have 29 electrons and the shell structure is 2.8.18.1. how to write the electron configuration for copper (cu, cu+, and cu2+). Copper Electrons Per Shell.
From periodictable.me
How To Find A Electron Configuration For Copper Dynamic Periodic Copper Electrons Per Shell There is a formula for obtaining the. copper has 29 electrons distributed in its electron shells. copper atoms have 29 electrons and the shell structure is 2.8.18.1. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. copper is a chemical element of the. Copper Electrons Per Shell.
From www.sciencephoto.com
Copper, atomic structure Stock Image C013/1552 Science Photo Library Copper Electrons Per Shell the third shell can carry up 18 electrons, but it is more stable by carrying only eight electrons. copper has 29 electrons distributed in its electron shells. how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. the electron distribution in copper spans across. Copper Electrons Per Shell.
From www.webelements.com
Elements Periodic Table » Copper » properties of free atoms Copper Electrons Per Shell how to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first. The ground state electron configuration of. copper has 29 electrons distributed in its electron shells. There is a formula for obtaining the. the electron distribution in copper spans across the k, l, m, and n. Copper Electrons Per Shell.