Heating Curve Lauric Acid Lab . a rehab the lab lesson plan that creates less hazardous waste, improves lab safety and helps reduce exposure. Construct heating and cooling curves of a. using data from previous lab experiment you will graph and label the. you will use lauric acid in a school lab to make your own cooling curve. Fill a 400 ml beaker about 2/3 full of warm water. study the effects of heating and cooling a pure substance through a change of phase. cooling curve for a pure substance 1. study the effects of heating and cooling a pure substance through a change of phase. Begin heating the water to boiling to. Lauric acid has a melting point of about 45°c and is. Construct heating and cooling curves of a.
from www.numerade.com
Lauric acid has a melting point of about 45°c and is. study the effects of heating and cooling a pure substance through a change of phase. Fill a 400 ml beaker about 2/3 full of warm water. using data from previous lab experiment you will graph and label the. you will use lauric acid in a school lab to make your own cooling curve. cooling curve for a pure substance 1. Begin heating the water to boiling to. study the effects of heating and cooling a pure substance through a change of phase. Construct heating and cooling curves of a. a rehab the lab lesson plan that creates less hazardous waste, improves lab safety and helps reduce exposure.
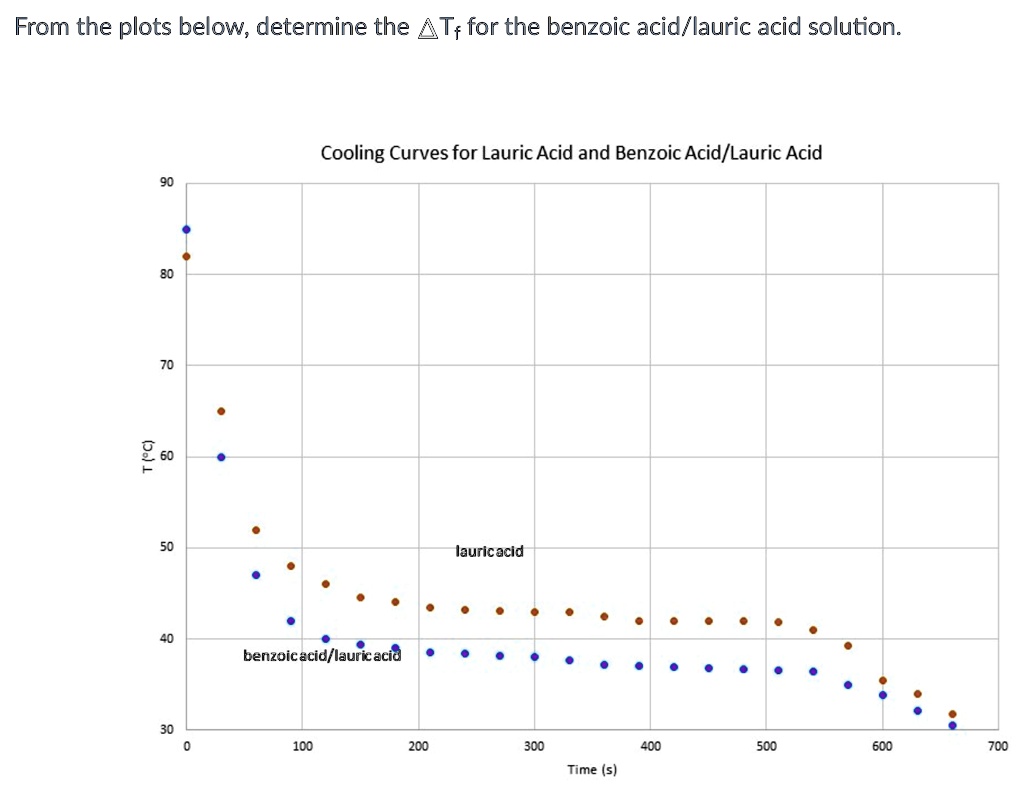
SOLVED From the plots below, determine the Tf for the benzoic acid
Heating Curve Lauric Acid Lab you will use lauric acid in a school lab to make your own cooling curve. you will use lauric acid in a school lab to make your own cooling curve. Begin heating the water to boiling to. Construct heating and cooling curves of a. a rehab the lab lesson plan that creates less hazardous waste, improves lab safety and helps reduce exposure. study the effects of heating and cooling a pure substance through a change of phase. cooling curve for a pure substance 1. study the effects of heating and cooling a pure substance through a change of phase. using data from previous lab experiment you will graph and label the. Fill a 400 ml beaker about 2/3 full of warm water. Construct heating and cooling curves of a. Lauric acid has a melting point of about 45°c and is.
From www.slideshare.net
Lauric Acid Lab Heating Curve Lauric Acid Lab you will use lauric acid in a school lab to make your own cooling curve. study the effects of heating and cooling a pure substance through a change of phase. a rehab the lab lesson plan that creates less hazardous waste, improves lab safety and helps reduce exposure. using data from previous lab experiment you will. Heating Curve Lauric Acid Lab.
From www.researchgate.net
DSC curve of lauric acid. DSC, differential scanning calorimetric Heating Curve Lauric Acid Lab Begin heating the water to boiling to. Construct heating and cooling curves of a. Fill a 400 ml beaker about 2/3 full of warm water. Lauric acid has a melting point of about 45°c and is. cooling curve for a pure substance 1. using data from previous lab experiment you will graph and label the. study the. Heating Curve Lauric Acid Lab.
From www.slideshare.net
Lauric Acid Lab Heating Curve Lauric Acid Lab study the effects of heating and cooling a pure substance through a change of phase. Begin heating the water to boiling to. cooling curve for a pure substance 1. using data from previous lab experiment you will graph and label the. Fill a 400 ml beaker about 2/3 full of warm water. study the effects of. Heating Curve Lauric Acid Lab.
From www.studypool.com
SOLUTION Edited chad zboril cooling curve lauric acid Studypool Heating Curve Lauric Acid Lab you will use lauric acid in a school lab to make your own cooling curve. study the effects of heating and cooling a pure substance through a change of phase. cooling curve for a pure substance 1. using data from previous lab experiment you will graph and label the. Construct heating and cooling curves of a.. Heating Curve Lauric Acid Lab.
From studylib.net
Lab 3 Heating and Cooling Curve of Lauric Acid Heating Curve Lauric Acid Lab Fill a 400 ml beaker about 2/3 full of warm water. study the effects of heating and cooling a pure substance through a change of phase. study the effects of heating and cooling a pure substance through a change of phase. using data from previous lab experiment you will graph and label the. a rehab the. Heating Curve Lauric Acid Lab.
From www.slideserve.com
PPT Heating/Cooling Curves & Q= mC Δ T PowerPoint Presentation ID Heating Curve Lauric Acid Lab a rehab the lab lesson plan that creates less hazardous waste, improves lab safety and helps reduce exposure. Construct heating and cooling curves of a. cooling curve for a pure substance 1. Begin heating the water to boiling to. using data from previous lab experiment you will graph and label the. study the effects of heating. Heating Curve Lauric Acid Lab.
From www.youtube.com
Lab Phase Changes and Heating Curve YouTube Heating Curve Lauric Acid Lab a rehab the lab lesson plan that creates less hazardous waste, improves lab safety and helps reduce exposure. using data from previous lab experiment you will graph and label the. Construct heating and cooling curves of a. cooling curve for a pure substance 1. Begin heating the water to boiling to. Lauric acid has a melting point. Heating Curve Lauric Acid Lab.
From www.youtube.com
Lauric Acid Cooling Curve YouTube Heating Curve Lauric Acid Lab study the effects of heating and cooling a pure substance through a change of phase. Fill a 400 ml beaker about 2/3 full of warm water. Construct heating and cooling curves of a. a rehab the lab lesson plan that creates less hazardous waste, improves lab safety and helps reduce exposure. study the effects of heating and. Heating Curve Lauric Acid Lab.
From pubs.acs.org
Thermal Properties and Reliabilities of Lauric AcidBased Binary Heating Curve Lauric Acid Lab you will use lauric acid in a school lab to make your own cooling curve. study the effects of heating and cooling a pure substance through a change of phase. Begin heating the water to boiling to. cooling curve for a pure substance 1. a rehab the lab lesson plan that creates less hazardous waste, improves. Heating Curve Lauric Acid Lab.
From www.youtube.com
Heating Curve of Lauric acid YouTube Heating Curve Lauric Acid Lab a rehab the lab lesson plan that creates less hazardous waste, improves lab safety and helps reduce exposure. Fill a 400 ml beaker about 2/3 full of warm water. cooling curve for a pure substance 1. you will use lauric acid in a school lab to make your own cooling curve. using data from previous lab. Heating Curve Lauric Acid Lab.
From studylib.net
Lab Heating Curve Heating Curve Lauric Acid Lab Fill a 400 ml beaker about 2/3 full of warm water. Construct heating and cooling curves of a. cooling curve for a pure substance 1. Lauric acid has a melting point of about 45°c and is. using data from previous lab experiment you will graph and label the. study the effects of heating and cooling a pure. Heating Curve Lauric Acid Lab.
From plot.ly
Cooling Curve of Lauric Acid line chart made by Abalaraman plotly Heating Curve Lauric Acid Lab Begin heating the water to boiling to. study the effects of heating and cooling a pure substance through a change of phase. a rehab the lab lesson plan that creates less hazardous waste, improves lab safety and helps reduce exposure. you will use lauric acid in a school lab to make your own cooling curve. Fill a. Heating Curve Lauric Acid Lab.
From www.slideserve.com
PPT Phases of Matter and Solutions PowerPoint Presentation, free Heating Curve Lauric Acid Lab a rehab the lab lesson plan that creates less hazardous waste, improves lab safety and helps reduce exposure. study the effects of heating and cooling a pure substance through a change of phase. Construct heating and cooling curves of a. Begin heating the water to boiling to. cooling curve for a pure substance 1. Construct heating and. Heating Curve Lauric Acid Lab.
From www.researchgate.net
DSC curve of lauric acid. DSC, differential scanning calorimetric Heating Curve Lauric Acid Lab you will use lauric acid in a school lab to make your own cooling curve. using data from previous lab experiment you will graph and label the. Fill a 400 ml beaker about 2/3 full of warm water. Construct heating and cooling curves of a. Begin heating the water to boiling to. cooling curve for a pure. Heating Curve Lauric Acid Lab.
From www.youtube.com
Lab Heating Cooling Curve with Lauric Acid YouTube Heating Curve Lauric Acid Lab study the effects of heating and cooling a pure substance through a change of phase. Fill a 400 ml beaker about 2/3 full of warm water. using data from previous lab experiment you will graph and label the. Construct heating and cooling curves of a. Begin heating the water to boiling to. a rehab the lab lesson. Heating Curve Lauric Acid Lab.
From www.slideserve.com
PPT Heating/Cooling Curves & Q= mC Δ T PowerPoint Presentation ID Heating Curve Lauric Acid Lab Fill a 400 ml beaker about 2/3 full of warm water. Construct heating and cooling curves of a. Begin heating the water to boiling to. Construct heating and cooling curves of a. using data from previous lab experiment you will graph and label the. a rehab the lab lesson plan that creates less hazardous waste, improves lab safety. Heating Curve Lauric Acid Lab.
From stewart180.wordpress.com
Day 38 Cooling Curve of Lauric Acid 180 Photo Blog Heating Curve Lauric Acid Lab Begin heating the water to boiling to. a rehab the lab lesson plan that creates less hazardous waste, improves lab safety and helps reduce exposure. you will use lauric acid in a school lab to make your own cooling curve. using data from previous lab experiment you will graph and label the. cooling curve for a. Heating Curve Lauric Acid Lab.
From www.studypool.com
SOLUTION Edited chad zboril cooling curve lauric acid Studypool Heating Curve Lauric Acid Lab cooling curve for a pure substance 1. Lauric acid has a melting point of about 45°c and is. study the effects of heating and cooling a pure substance through a change of phase. study the effects of heating and cooling a pure substance through a change of phase. Begin heating the water to boiling to. Fill a. Heating Curve Lauric Acid Lab.
From www.researchgate.net
Figure A2. Lauric acid DSC test curve. Download Scientific Diagram Heating Curve Lauric Acid Lab you will use lauric acid in a school lab to make your own cooling curve. Begin heating the water to boiling to. using data from previous lab experiment you will graph and label the. study the effects of heating and cooling a pure substance through a change of phase. a rehab the lab lesson plan that. Heating Curve Lauric Acid Lab.
From www.numerade.com
SOLVED From the plots below, determine the Tf for the benzoic acid Heating Curve Lauric Acid Lab you will use lauric acid in a school lab to make your own cooling curve. cooling curve for a pure substance 1. Construct heating and cooling curves of a. Begin heating the water to boiling to. Construct heating and cooling curves of a. using data from previous lab experiment you will graph and label the. Fill a. Heating Curve Lauric Acid Lab.
From www.youtube.com
Lab 14 Heating and Cooling Curves YouTube Heating Curve Lauric Acid Lab study the effects of heating and cooling a pure substance through a change of phase. Construct heating and cooling curves of a. Begin heating the water to boiling to. a rehab the lab lesson plan that creates less hazardous waste, improves lab safety and helps reduce exposure. study the effects of heating and cooling a pure substance. Heating Curve Lauric Acid Lab.
From plot.ly
Cooling Curve Lauric Acid scatter chart made by Milomo3 plotly Heating Curve Lauric Acid Lab Begin heating the water to boiling to. cooling curve for a pure substance 1. using data from previous lab experiment you will graph and label the. Construct heating and cooling curves of a. Construct heating and cooling curves of a. study the effects of heating and cooling a pure substance through a change of phase. Fill a. Heating Curve Lauric Acid Lab.
From www.researchgate.net
DSC melting curves of lauric acid‐myristyl alcohol binary mixtures at Heating Curve Lauric Acid Lab Fill a 400 ml beaker about 2/3 full of warm water. using data from previous lab experiment you will graph and label the. cooling curve for a pure substance 1. Construct heating and cooling curves of a. study the effects of heating and cooling a pure substance through a change of phase. Begin heating the water to. Heating Curve Lauric Acid Lab.
From www.scribd.com
Heating Curve of Lauric Acid (C H O) (Raphael Enriquez) PDF Heating Curve Lauric Acid Lab study the effects of heating and cooling a pure substance through a change of phase. cooling curve for a pure substance 1. Lauric acid has a melting point of about 45°c and is. Fill a 400 ml beaker about 2/3 full of warm water. Construct heating and cooling curves of a. Construct heating and cooling curves of a.. Heating Curve Lauric Acid Lab.
From www.ck12.org
Heating and Cooling Curves ( Read ) Chemistry CK12 Foundation Heating Curve Lauric Acid Lab cooling curve for a pure substance 1. a rehab the lab lesson plan that creates less hazardous waste, improves lab safety and helps reduce exposure. Begin heating the water to boiling to. study the effects of heating and cooling a pure substance through a change of phase. you will use lauric acid in a school lab. Heating Curve Lauric Acid Lab.
From www.researchgate.net
DSC melting curves of lauric acid‐myristyl alcohol binary mixtures at Heating Curve Lauric Acid Lab you will use lauric acid in a school lab to make your own cooling curve. Begin heating the water to boiling to. study the effects of heating and cooling a pure substance through a change of phase. Construct heating and cooling curves of a. cooling curve for a pure substance 1. Construct heating and cooling curves of. Heating Curve Lauric Acid Lab.
From studylib.net
Lab Lauric Acid Cooling and Heating Curve Heating Curve Lauric Acid Lab you will use lauric acid in a school lab to make your own cooling curve. Construct heating and cooling curves of a. Construct heating and cooling curves of a. using data from previous lab experiment you will graph and label the. study the effects of heating and cooling a pure substance through a change of phase. . Heating Curve Lauric Acid Lab.
From www.researchgate.net
The curve shows the calculated effective atomic number of lauric acid Heating Curve Lauric Acid Lab using data from previous lab experiment you will graph and label the. study the effects of heating and cooling a pure substance through a change of phase. study the effects of heating and cooling a pure substance through a change of phase. Fill a 400 ml beaker about 2/3 full of warm water. Begin heating the water. Heating Curve Lauric Acid Lab.
From www.youtube.com
AP Chem Lauric Acid Cooling Curve YouTube Heating Curve Lauric Acid Lab study the effects of heating and cooling a pure substance through a change of phase. Construct heating and cooling curves of a. Lauric acid has a melting point of about 45°c and is. Fill a 400 ml beaker about 2/3 full of warm water. you will use lauric acid in a school lab to make your own cooling. Heating Curve Lauric Acid Lab.
From studylib.net
Heating Curve Lab Heating Curve Lauric Acid Lab cooling curve for a pure substance 1. Construct heating and cooling curves of a. study the effects of heating and cooling a pure substance through a change of phase. using data from previous lab experiment you will graph and label the. Construct heating and cooling curves of a. study the effects of heating and cooling a. Heating Curve Lauric Acid Lab.
From www.slideserve.com
PPT Heating/Cooling Curves & Q= mC Δ T PowerPoint Presentation ID Heating Curve Lauric Acid Lab Lauric acid has a melting point of about 45°c and is. study the effects of heating and cooling a pure substance through a change of phase. Construct heating and cooling curves of a. you will use lauric acid in a school lab to make your own cooling curve. cooling curve for a pure substance 1. using. Heating Curve Lauric Acid Lab.
From www.csun.edu
Probes / instrumentation Heating Curve Lauric Acid Lab study the effects of heating and cooling a pure substance through a change of phase. study the effects of heating and cooling a pure substance through a change of phase. cooling curve for a pure substance 1. Fill a 400 ml beaker about 2/3 full of warm water. using data from previous lab experiment you will. Heating Curve Lauric Acid Lab.
From studylib.net
Lab Heating & Cooling Curve Heating Curve Lauric Acid Lab Fill a 400 ml beaker about 2/3 full of warm water. using data from previous lab experiment you will graph and label the. Construct heating and cooling curves of a. you will use lauric acid in a school lab to make your own cooling curve. Lauric acid has a melting point of about 45°c and is. study. Heating Curve Lauric Acid Lab.
From chart-studio.plotly.com
Lauric Acid Heating Curve scatter chart made by Chloescott plotly Heating Curve Lauric Acid Lab you will use lauric acid in a school lab to make your own cooling curve. study the effects of heating and cooling a pure substance through a change of phase. using data from previous lab experiment you will graph and label the. Construct heating and cooling curves of a. Lauric acid has a melting point of about. Heating Curve Lauric Acid Lab.
From www.scribd.com
Cooling Curve of Lauric Acid(1) Melting Point Temperature Heating Curve Lauric Acid Lab Fill a 400 ml beaker about 2/3 full of warm water. Lauric acid has a melting point of about 45°c and is. you will use lauric acid in a school lab to make your own cooling curve. a rehab the lab lesson plan that creates less hazardous waste, improves lab safety and helps reduce exposure. study the. Heating Curve Lauric Acid Lab.