Standard Cell Potential Questions . Only the difference between the potentials of two electrodes can be measured. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. To find the difference of the two. \begin {align*}e^0_ {\text {ag}}=+0.80 \text {. the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called the standard cell potential (e° cell). List the known values and plan the problem. this chemistry video explains how to calculate the standard cell potential of a galvanic cell and an electrolytic cell. the standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams.
from www.chegg.com
List the known values and plan the problem. To find the difference of the two. the standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. \begin {align*}e^0_ {\text {ag}}=+0.80 \text {. this chemistry video explains how to calculate the standard cell potential of a galvanic cell and an electrolytic cell. revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. Only the difference between the potentials of two electrodes can be measured. the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called the standard cell potential (e° cell).
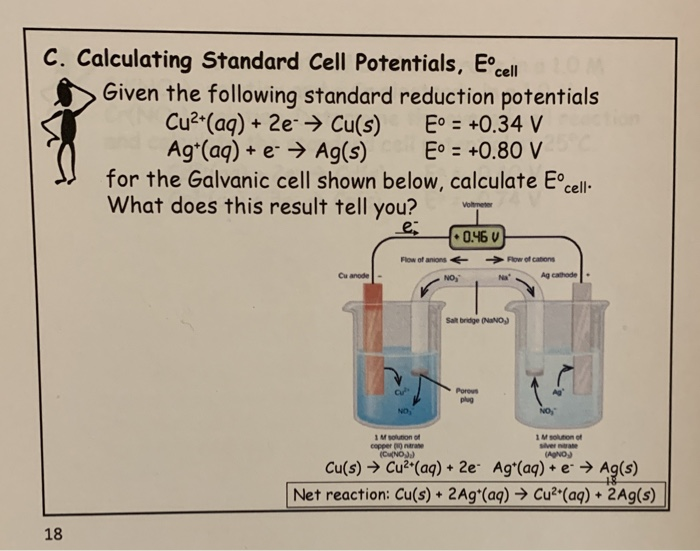
Solved C. Calculating Standard Cell Potentials, Eºcell Given
Standard Cell Potential Questions this chemistry video explains how to calculate the standard cell potential of a galvanic cell and an electrolytic cell. \begin {align*}e^0_ {\text {ag}}=+0.80 \text {. List the known values and plan the problem. Only the difference between the potentials of two electrodes can be measured. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. To find the difference of the two. the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called the standard cell potential (e° cell). this chemistry video explains how to calculate the standard cell potential of a galvanic cell and an electrolytic cell. the standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell.
From www.chegg.com
Solved Calculate the standard cell potential for each of the Standard Cell Potential Questions 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. To find the difference of the two. this chemistry video explains how to calculate the standard cell potential of a galvanic cell and an electrolytic cell. the standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the. Standard Cell Potential Questions.
From www.coursehero.com
[Solved] The standard cell potential (E.cell) of the reaction below is Standard Cell Potential Questions To find the difference of the two. revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. \begin {align*}e^0_ {\text {ag}}=+0.80 \text {. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. List the known values. Standard Cell Potential Questions.
From www.chegg.com
Solved QUESTION 2 The standard cell potential E for the cell Standard Cell Potential Questions Only the difference between the potentials of two electrodes can be measured. this chemistry video explains how to calculate the standard cell potential of a galvanic cell and an electrolytic cell. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. List the known values and plan the problem. the standard. Standard Cell Potential Questions.
From general.chemistrysteps.com
How to Calculate Standard Cell Potential Chemistry Steps Standard Cell Potential Questions 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. Only the difference between the potentials of two electrodes can be measured. this chemistry video explains how to calculate the standard cell potential of a galvanic cell and an electrolytic cell. To find the difference of the two. List the known values. Standard Cell Potential Questions.
From oneclass.com
OneClass The standard cell potential(E Standard Cell Potential Questions To find the difference of the two. this chemistry video explains how to calculate the standard cell potential of a galvanic cell and an electrolytic cell. \begin {align*}e^0_ {\text {ag}}=+0.80 \text {. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. the standard cell potential (\(e^o_{cell}\)) is the difference of. Standard Cell Potential Questions.
From www.numerade.com
SOLVED Calculate the standard cell potential at 25 for the reaction X Standard Cell Potential Questions Only the difference between the potentials of two electrodes can be measured. \begin {align*}e^0_ {\text {ag}}=+0.80 \text {. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. List the known values and plan the problem. the standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage. Standard Cell Potential Questions.
From www.chegg.com
Solved Determine the standard cell potential for each of the Standard Cell Potential Questions 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called the standard cell potential (e° cell). Only the difference between the. Standard Cell Potential Questions.
From www.chegg.com
Solved Calculate the standard cell potential, E., for the Standard Cell Potential Questions List the known values and plan the problem. the standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. Standard Cell Potential Questions.
From www.chegg.com
Solved Use the halfreactions below to produce a voltaic Standard Cell Potential Questions the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called the standard cell potential (e° cell). 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. revision notes on 5.3.3. Standard Cell Potential Questions.
From www.chegg.com
Solved The standard cell potential, E°, for the reaction Standard Cell Potential Questions the standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. Only the difference between the potentials of two electrodes can be measured. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. this chemistry video explains how to calculate the standard cell. Standard Cell Potential Questions.
From www.chegg.com
Solved QUESTION 5 The standard cell potential (Eºcell ) for Standard Cell Potential Questions To find the difference of the two. \begin {align*}e^0_ {\text {ag}}=+0.80 \text {. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. List the known values and plan the problem. the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for. Standard Cell Potential Questions.
From www.chegg.com
Solved The standard cell potential (Eºcell) for the voltaic Standard Cell Potential Questions To find the difference of the two. \begin {align*}e^0_ {\text {ag}}=+0.80 \text {. List the known values and plan the problem. Only the difference between the potentials of two electrodes can be measured. revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. . Standard Cell Potential Questions.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Standard Cell Potential Questions \begin {align*}e^0_ {\text {ag}}=+0.80 \text {. revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. List the known values and plan the problem. Only the difference between the potentials of two electrodes can be measured. 42 rows calculate the standard cell potential. Standard Cell Potential Questions.
From www.chegg.com
Solved C. Calculating Standard Cell Potentials, Eºcell Given Standard Cell Potential Questions To find the difference of the two. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. \begin {align*}e^0_ {\text {ag}}=+0.80 \text {. this chemistry video explains how to calculate the standard cell potential of a galvanic cell and an electrolytic cell. the potential of the cell under standard conditions (1. Standard Cell Potential Questions.
From www.chegg.com
Solved The standard cell potential (E cell) for the reaction Standard Cell Potential Questions 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. this chemistry video explains how to calculate the standard cell potential of a galvanic cell and an electrolytic cell. To find the difference of the two. Only the difference between the potentials of two electrodes can be measured. \begin {align*}e^0_ {\text {ag}}=+0.80. Standard Cell Potential Questions.
From www.chegg.com
Solved Calculate the standard cell potential at 25 degree C Standard Cell Potential Questions Only the difference between the potentials of two electrodes can be measured. this chemistry video explains how to calculate the standard cell potential of a galvanic cell and an electrolytic cell. the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed. Standard Cell Potential Questions.
From www.chegg.com
Solved A. Calculate the standard cell potential (Eºcell) for Standard Cell Potential Questions \begin {align*}e^0_ {\text {ag}}=+0.80 \text {. this chemistry video explains how to calculate the standard cell potential of a galvanic cell and an electrolytic cell. revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. To find the difference of the two. . Standard Cell Potential Questions.
From www.coursehero.com
[Solved] The Standard cell potential E cell, for the electrochemical Standard Cell Potential Questions the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called the standard cell potential (e° cell). List the known values and plan the problem. 42 rows calculate the standard cell potential of a voltaic cell that uses. Standard Cell Potential Questions.
From www.slideserve.com
PPT Chemistry 30 Chapter 14 PowerPoint Presentation, free download Standard Cell Potential Questions the standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called the standard cell potential (e° cell). revision. Standard Cell Potential Questions.
From kunduz.com
[ANSWERED] Calculate the standard cell potential, E cell' for the Kunduz Standard Cell Potential Questions revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. To find the difference of the two. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. \begin {align*}e^0_ {\text {ag}}=+0.80 \text {. List the known values. Standard Cell Potential Questions.
From www.numerade.com
SOLVED 1 Calculate the standard cell potential (from the Standard Standard Cell Potential Questions the standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. List the known values and plan the problem. the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. Standard Cell Potential Questions.
From www.chegg.com
Solved Calculate the standard cell potential for each of the Standard Cell Potential Questions List the known values and plan the problem. \begin {align*}e^0_ {\text {ag}}=+0.80 \text {. revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or. Standard Cell Potential Questions.
From askfilo.com
93. Calculate the standard cell potential (in volt) of the cell in which Standard Cell Potential Questions \begin {align*}e^0_ {\text {ag}}=+0.80 \text {. the standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. To find the difference of the two. . Standard Cell Potential Questions.
From www.bartleby.com
Answered The standard cell potential (E° cell)… bartleby Standard Cell Potential Questions the standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. the potential of the cell under standard conditions (1 m for solutions, 1. Standard Cell Potential Questions.
From www.bartleby.com
Answered B) Calculate standard cell potential… bartleby Standard Cell Potential Questions 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. Only the difference between the potentials of two electrodes can be measured. To find the difference of the two. List the known values and plan the problem. this chemistry video explains how to calculate the standard cell potential of a galvanic cell. Standard Cell Potential Questions.
From www.nagwa.com
Question Video Calculating the Standard Cell Potential for a Magnesium Standard Cell Potential Questions this chemistry video explains how to calculate the standard cell potential of a galvanic cell and an electrolytic cell. List the known values and plan the problem. \begin {align*}e^0_ {\text {ag}}=+0.80 \text {. Only the difference between the potentials of two electrodes can be measured. the potential of the cell under standard conditions (1 m for solutions, 1. Standard Cell Potential Questions.
From www.chegg.com
Solved 5. Calculate the standard cell potential for the Standard Cell Potential Questions this chemistry video explains how to calculate the standard cell potential of a galvanic cell and an electrolytic cell. Only the difference between the potentials of two electrodes can be measured. \begin {align*}e^0_ {\text {ag}}=+0.80 \text {. 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\) and. the potential of the. Standard Cell Potential Questions.
From www.chegg.com
Solved Using the Standard Cell Potential Equation, Ecell = Standard Cell Potential Questions the standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. Only the difference between the potentials of two electrodes can be measured. 42. Standard Cell Potential Questions.
From www.bartleby.com
Answered 31)Calculate the cell potential for the… bartleby Standard Cell Potential Questions revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts at save my exams. Only the difference between the potentials of two electrodes can be measured. List the known values and plan the problem. To find the difference of the two. this chemistry video explains how to. Standard Cell Potential Questions.
From thechemistrynotes.com
Cell Potential & Standard Cell Potential Standard Cell Potential Questions the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called the standard cell potential (e° cell). the standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. Only the. Standard Cell Potential Questions.
From www.chegg.com
Solved Calculate the standard cell potential for the Standard Cell Potential Questions To find the difference of the two. the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called the standard cell potential (e° cell). Only the difference between the potentials of two electrodes can be measured. revision notes. Standard Cell Potential Questions.
From www.chegg.com
Solved Calculate the standard cell potential of the Standard Cell Potential Questions \begin {align*}e^0_ {\text {ag}}=+0.80 \text {. the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called the standard cell potential (e° cell). To find the difference of the two. Only the difference between the potentials of two electrodes. Standard Cell Potential Questions.
From www.coursehero.com
Determine the standard cell potential for the reaction below of Standard Cell Potential Questions the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called the standard cell potential (e° cell). \begin {align*}e^0_ {\text {ag}}=+0.80 \text {. List the known values and plan the problem. To find the difference of the two. . Standard Cell Potential Questions.
From www.chegg.com
Solved Question 3 (2 points) The standard cell potential of Standard Cell Potential Questions \begin {align*}e^0_ {\text {ag}}=+0.80 \text {. the potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called the standard cell potential (e° cell). 42 rows calculate the standard cell potential of a voltaic cell that uses the \(\ce{ag}\)/\(\ce{ag^+}\). Standard Cell Potential Questions.
From www.chegg.com
Solved The standard cell potential (E^0 cell) for the Standard Cell Potential Questions Only the difference between the potentials of two electrodes can be measured. To find the difference of the two. the standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry. Standard Cell Potential Questions.