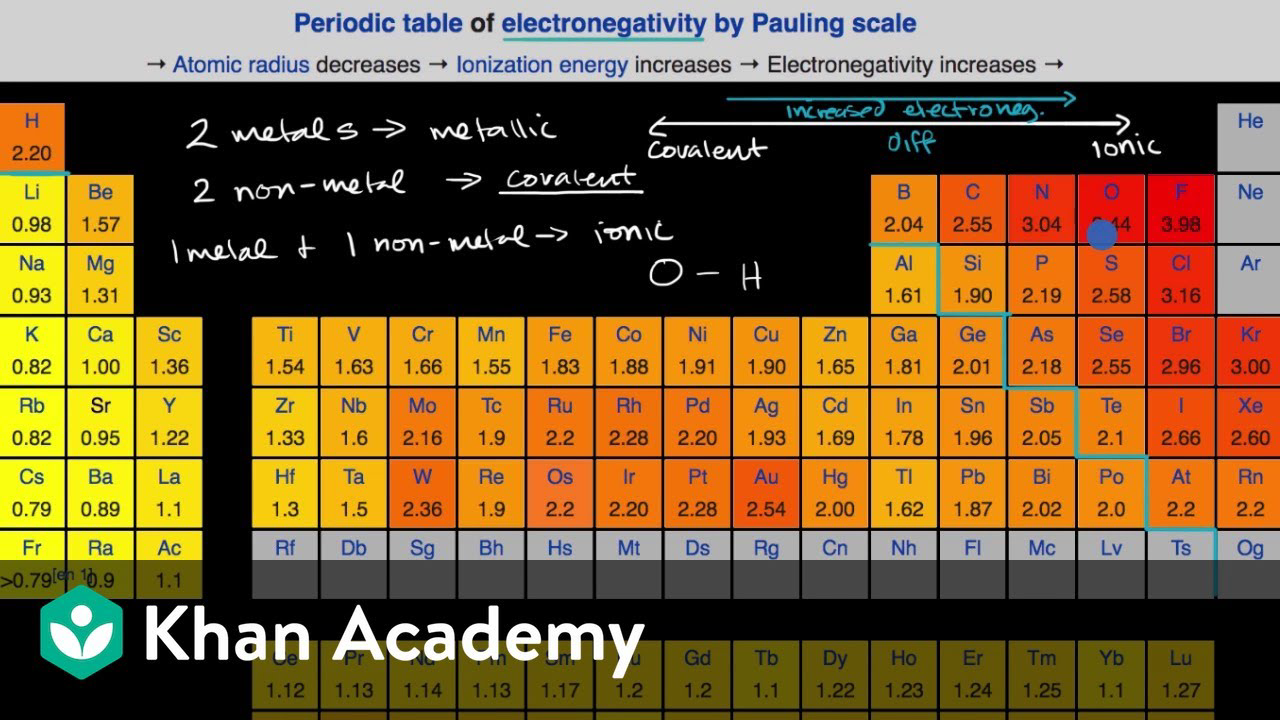
Cobalt Bromide Electronegativity Difference . — the electronegativity calculator allows you to calculate the type of bond formed between different elements using their. — differences in electronegativity between two atoms can be used to determine if their bond is nonpolar, polar, or ionic. The suggested values are all. In a diatomic molecule with two identical atoms, there is. 103 rows — electronegativity is not a uniquely defined property and may depend on the definition. you can compare co vs br on more than 90 properties like electronegativity , oxidation state, atomic shells, orbital structure,. In a diatomic molecule with two. 119 rows — — find the electronegativity values of all elements in a table, including cobalt (co) with 1.88 and bromine. the difference in electronegativity between two atoms determines how polar a bond will be. the difference in electronegativity between two atoms determines how polar a bond will be.
from www.animalia-life.club
In a diatomic molecule with two identical atoms, there is. the difference in electronegativity between two atoms determines how polar a bond will be. — the electronegativity calculator allows you to calculate the type of bond formed between different elements using their. you can compare co vs br on more than 90 properties like electronegativity , oxidation state, atomic shells, orbital structure,. — differences in electronegativity between two atoms can be used to determine if their bond is nonpolar, polar, or ionic. 119 rows — — find the electronegativity values of all elements in a table, including cobalt (co) with 1.88 and bromine. In a diatomic molecule with two. The suggested values are all. 103 rows — electronegativity is not a uniquely defined property and may depend on the definition. the difference in electronegativity between two atoms determines how polar a bond will be.
Electronegativity Difference Bond Type
Cobalt Bromide Electronegativity Difference the difference in electronegativity between two atoms determines how polar a bond will be. the difference in electronegativity between two atoms determines how polar a bond will be. In a diatomic molecule with two. In a diatomic molecule with two identical atoms, there is. — differences in electronegativity between two atoms can be used to determine if their bond is nonpolar, polar, or ionic. — the electronegativity calculator allows you to calculate the type of bond formed between different elements using their. 119 rows — — find the electronegativity values of all elements in a table, including cobalt (co) with 1.88 and bromine. 103 rows — electronegativity is not a uniquely defined property and may depend on the definition. you can compare co vs br on more than 90 properties like electronegativity , oxidation state, atomic shells, orbital structure,. the difference in electronegativity between two atoms determines how polar a bond will be. The suggested values are all.
From www.dreamstime.com
3D Image of Vinyl Bromide Skeletal Formula Stock Illustration Cobalt Bromide Electronegativity Difference In a diatomic molecule with two identical atoms, there is. The suggested values are all. you can compare co vs br on more than 90 properties like electronegativity , oxidation state, atomic shells, orbital structure,. the difference in electronegativity between two atoms determines how polar a bond will be. 119 rows — — find the electronegativity values. Cobalt Bromide Electronegativity Difference.
From mungfali.com
Electronegativity Difference Chart Cobalt Bromide Electronegativity Difference In a diatomic molecule with two identical atoms, there is. the difference in electronegativity between two atoms determines how polar a bond will be. you can compare co vs br on more than 90 properties like electronegativity , oxidation state, atomic shells, orbital structure,. the difference in electronegativity between two atoms determines how polar a bond will. Cobalt Bromide Electronegativity Difference.
From www.dreamstime.com
Hydrogen Bromide HBr Molecule. Skeletal Formula. Stock Vector Cobalt Bromide Electronegativity Difference — the electronegativity calculator allows you to calculate the type of bond formed between different elements using their. — differences in electronegativity between two atoms can be used to determine if their bond is nonpolar, polar, or ionic. the difference in electronegativity between two atoms determines how polar a bond will be. In a diatomic molecule with. Cobalt Bromide Electronegativity Difference.
From www.animalia-life.club
Electronegativity Difference Bond Type Cobalt Bromide Electronegativity Difference In a diatomic molecule with two identical atoms, there is. The suggested values are all. 119 rows — — find the electronegativity values of all elements in a table, including cobalt (co) with 1.88 and bromine. — differences in electronegativity between two atoms can be used to determine if their bond is nonpolar, polar, or ionic. you. Cobalt Bromide Electronegativity Difference.
From www.alamy.com
Tiotropium bromide chronic obstructive pulmonary disease (COPD) drug Cobalt Bromide Electronegativity Difference 119 rows — — find the electronegativity values of all elements in a table, including cobalt (co) with 1.88 and bromine. In a diatomic molecule with two identical atoms, there is. The suggested values are all. you can compare co vs br on more than 90 properties like electronegativity , oxidation state, atomic shells, orbital structure,. In a. Cobalt Bromide Electronegativity Difference.
From www.vectorstock.com
Ch3br methyl bromide molecule Royalty Free Vector Image Cobalt Bromide Electronegativity Difference The suggested values are all. 119 rows — — find the electronegativity values of all elements in a table, including cobalt (co) with 1.88 and bromine. In a diatomic molecule with two identical atoms, there is. In a diatomic molecule with two. 103 rows — electronegativity is not a uniquely defined property and may depend on the definition.. Cobalt Bromide Electronegativity Difference.
From mavink.com
Electronegativity Difference Chart Cobalt Bromide Electronegativity Difference 119 rows — — find the electronegativity values of all elements in a table, including cobalt (co) with 1.88 and bromine. you can compare co vs br on more than 90 properties like electronegativity , oxidation state, atomic shells, orbital structure,. the difference in electronegativity between two atoms determines how polar a bond will be. In a. Cobalt Bromide Electronegativity Difference.
From us.metoree.com
41 Ethyl Bromide Manufacturers in 2024 Metoree Cobalt Bromide Electronegativity Difference the difference in electronegativity between two atoms determines how polar a bond will be. — the electronegativity calculator allows you to calculate the type of bond formed between different elements using their. In a diatomic molecule with two. — differences in electronegativity between two atoms can be used to determine if their bond is nonpolar, polar, or. Cobalt Bromide Electronegativity Difference.
From www.animalia-life.club
Electronegativity Difference Bond Type Cobalt Bromide Electronegativity Difference the difference in electronegativity between two atoms determines how polar a bond will be. 119 rows — — find the electronegativity values of all elements in a table, including cobalt (co) with 1.88 and bromine. 103 rows — electronegativity is not a uniquely defined property and may depend on the definition. In a diatomic molecule with two. Cobalt Bromide Electronegativity Difference.
From www.alamy.com
Glycopyrronium bromide (glycopyrrolate) COPD drug molecule. Has Cobalt Bromide Electronegativity Difference 103 rows — electronegativity is not a uniquely defined property and may depend on the definition. — the electronegativity calculator allows you to calculate the type of bond formed between different elements using their. — differences in electronegativity between two atoms can be used to determine if their bond is nonpolar, polar, or ionic. In a diatomic. Cobalt Bromide Electronegativity Difference.
From byjus.com
Mechanism of Hoffman bromide degradation reaction Cobalt Bromide Electronegativity Difference In a diatomic molecule with two identical atoms, there is. 103 rows — electronegativity is not a uniquely defined property and may depend on the definition. 119 rows — — find the electronegativity values of all elements in a table, including cobalt (co) with 1.88 and bromine. — differences in electronegativity between two atoms can be used. Cobalt Bromide Electronegativity Difference.
From www.alamy.com
Gel electrophoresis ethidium bromide hires stock photography and Cobalt Bromide Electronegativity Difference the difference in electronegativity between two atoms determines how polar a bond will be. — differences in electronegativity between two atoms can be used to determine if their bond is nonpolar, polar, or ionic. you can compare co vs br on more than 90 properties like electronegativity , oxidation state, atomic shells, orbital structure,. — the. Cobalt Bromide Electronegativity Difference.
From brainly.com
Which particle represents the size of the bromide ion compared to the Cobalt Bromide Electronegativity Difference the difference in electronegativity between two atoms determines how polar a bond will be. the difference in electronegativity between two atoms determines how polar a bond will be. 119 rows — — find the electronegativity values of all elements in a table, including cobalt (co) with 1.88 and bromine. In a diatomic molecule with two. —. Cobalt Bromide Electronegativity Difference.
From hamptonresearch.com
Hampton Research Cobalt Bromide Electronegativity Difference In a diatomic molecule with two identical atoms, there is. the difference in electronegativity between two atoms determines how polar a bond will be. — differences in electronegativity between two atoms can be used to determine if their bond is nonpolar, polar, or ionic. — the electronegativity calculator allows you to calculate the type of bond formed. Cobalt Bromide Electronegativity Difference.
From www.researchgate.net
Top Chemical structures of CPEs and salts tetraethylammonium bromide Cobalt Bromide Electronegativity Difference 119 rows — — find the electronegativity values of all elements in a table, including cobalt (co) with 1.88 and bromine. you can compare co vs br on more than 90 properties like electronegativity , oxidation state, atomic shells, orbital structure,. 103 rows — electronegativity is not a uniquely defined property and may depend on the definition.. Cobalt Bromide Electronegativity Difference.
From www.chemistryworld.com
Potassium bromide Podcast Chemistry World Cobalt Bromide Electronegativity Difference the difference in electronegativity between two atoms determines how polar a bond will be. you can compare co vs br on more than 90 properties like electronegativity , oxidation state, atomic shells, orbital structure,. The suggested values are all. In a diatomic molecule with two identical atoms, there is. — differences in electronegativity between two atoms can. Cobalt Bromide Electronegativity Difference.
From testbook.com
Lithium Bromide Learn Structure, Properties, Preparation & Uses. Cobalt Bromide Electronegativity Difference — differences in electronegativity between two atoms can be used to determine if their bond is nonpolar, polar, or ionic. 119 rows — — find the electronegativity values of all elements in a table, including cobalt (co) with 1.88 and bromine. In a diatomic molecule with two. the difference in electronegativity between two atoms determines how polar. Cobalt Bromide Electronegativity Difference.
From www.dreamstime.com
Potassium Bromide Chemical Formula on Waterdrop Background Stock Cobalt Bromide Electronegativity Difference The suggested values are all. In a diatomic molecule with two. — differences in electronegativity between two atoms can be used to determine if their bond is nonpolar, polar, or ionic. In a diatomic molecule with two identical atoms, there is. 103 rows — electronegativity is not a uniquely defined property and may depend on the definition. . Cobalt Bromide Electronegativity Difference.
From www.bhphotovideo.com
Photographers' Formulary Potassium Bromide (100g) 100930 100G Cobalt Bromide Electronegativity Difference 119 rows — — find the electronegativity values of all elements in a table, including cobalt (co) with 1.88 and bromine. — differences in electronegativity between two atoms can be used to determine if their bond is nonpolar, polar, or ionic. 103 rows — electronegativity is not a uniquely defined property and may depend on the definition.. Cobalt Bromide Electronegativity Difference.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine Cobalt Bromide Electronegativity Difference — differences in electronegativity between two atoms can be used to determine if their bond is nonpolar, polar, or ionic. 103 rows — electronegativity is not a uniquely defined property and may depend on the definition. The suggested values are all. 119 rows — — find the electronegativity values of all elements in a table, including cobalt. Cobalt Bromide Electronegativity Difference.
From www.shutterstock.com
Sodium Bromide Properties Chemical Compound Structure Stock Vector Cobalt Bromide Electronegativity Difference — differences in electronegativity between two atoms can be used to determine if their bond is nonpolar, polar, or ionic. you can compare co vs br on more than 90 properties like electronegativity , oxidation state, atomic shells, orbital structure,. In a diatomic molecule with two identical atoms, there is. 103 rows — electronegativity is not a. Cobalt Bromide Electronegativity Difference.
From mungfali.com
Electronegativity Difference Chart Cobalt Bromide Electronegativity Difference In a diatomic molecule with two. 119 rows — — find the electronegativity values of all elements in a table, including cobalt (co) with 1.88 and bromine. the difference in electronegativity between two atoms determines how polar a bond will be. 103 rows — electronegativity is not a uniquely defined property and may depend on the definition.. Cobalt Bromide Electronegativity Difference.
From www.rpicorp.com
E718005.0 Ethidium Bromide, Powder, 5 Grams Cobalt Bromide Electronegativity Difference the difference in electronegativity between two atoms determines how polar a bond will be. 103 rows — electronegativity is not a uniquely defined property and may depend on the definition. The suggested values are all. In a diatomic molecule with two identical atoms, there is. In a diatomic molecule with two. the difference in electronegativity between two. Cobalt Bromide Electronegativity Difference.
From sielc.com
Vinyl bromide SIELC Technologies Cobalt Bromide Electronegativity Difference — the electronegativity calculator allows you to calculate the type of bond formed between different elements using their. In a diatomic molecule with two. In a diatomic molecule with two identical atoms, there is. — differences in electronegativity between two atoms can be used to determine if their bond is nonpolar, polar, or ionic. The suggested values are. Cobalt Bromide Electronegativity Difference.
From www.macsenlab.com
Pinaverium Bromide API 53251948 Manufacturer & Supplier Cobalt Bromide Electronegativity Difference 103 rows — electronegativity is not a uniquely defined property and may depend on the definition. — the electronegativity calculator allows you to calculate the type of bond formed between different elements using their. In a diatomic molecule with two identical atoms, there is. 119 rows — — find the electronegativity values of all elements in a. Cobalt Bromide Electronegativity Difference.
From www.chemkits.eu
Sodium bromide, 99.8+, 7647156 Cobalt Bromide Electronegativity Difference — differences in electronegativity between two atoms can be used to determine if their bond is nonpolar, polar, or ionic. In a diatomic molecule with two. 103 rows — electronegativity is not a uniquely defined property and may depend on the definition. The suggested values are all. In a diatomic molecule with two identical atoms, there is. . Cobalt Bromide Electronegativity Difference.
From dxovxnlin.blob.core.windows.net
Bromide Anion Has How Many Electrons at Emma Stinson blog Cobalt Bromide Electronegativity Difference you can compare co vs br on more than 90 properties like electronegativity , oxidation state, atomic shells, orbital structure,. The suggested values are all. In a diatomic molecule with two identical atoms, there is. In a diatomic molecule with two. 119 rows — — find the electronegativity values of all elements in a table, including cobalt (co). Cobalt Bromide Electronegativity Difference.
From sielc.com
Ethidium bromide SIELC Technologies Cobalt Bromide Electronegativity Difference 103 rows — electronegativity is not a uniquely defined property and may depend on the definition. In a diatomic molecule with two identical atoms, there is. — differences in electronegativity between two atoms can be used to determine if their bond is nonpolar, polar, or ionic. — the electronegativity calculator allows you to calculate the type of. Cobalt Bromide Electronegativity Difference.
From cartoondealer.com
Ipratropium Bromide Molecule, Molecular Structures, Atrovent, 3d Model Cobalt Bromide Electronegativity Difference 119 rows — — find the electronegativity values of all elements in a table, including cobalt (co) with 1.88 and bromine. — differences in electronegativity between two atoms can be used to determine if their bond is nonpolar, polar, or ionic. In a diatomic molecule with two. the difference in electronegativity between two atoms determines how polar. Cobalt Bromide Electronegativity Difference.
From www.numerade.com
There are four alkyl bromides with the formula C 4 H9 Br. Write their Cobalt Bromide Electronegativity Difference The suggested values are all. — the electronegativity calculator allows you to calculate the type of bond formed between different elements using their. 103 rows — electronegativity is not a uniquely defined property and may depend on the definition. — differences in electronegativity between two atoms can be used to determine if their bond is nonpolar, polar,. Cobalt Bromide Electronegativity Difference.
From byjus.com
Briefly explain the process of electrolysis of molten Lead Bromide Cobalt Bromide Electronegativity Difference you can compare co vs br on more than 90 properties like electronegativity , oxidation state, atomic shells, orbital structure,. the difference in electronegativity between two atoms determines how polar a bond will be. 119 rows — — find the electronegativity values of all elements in a table, including cobalt (co) with 1.88 and bromine. —. Cobalt Bromide Electronegativity Difference.
From www.chemistrylearner.com
Sodium Bromate Facts, Formula, Properties, Uses, Safety Data Cobalt Bromide Electronegativity Difference In a diatomic molecule with two. the difference in electronegativity between two atoms determines how polar a bond will be. — differences in electronegativity between two atoms can be used to determine if their bond is nonpolar, polar, or ionic. you can compare co vs br on more than 90 properties like electronegativity , oxidation state, atomic. Cobalt Bromide Electronegativity Difference.
From byjus.com
Write the molecular formulae for the compound Copper II Bromide Cobalt Bromide Electronegativity Difference In a diatomic molecule with two. 103 rows — electronegativity is not a uniquely defined property and may depend on the definition. — differences in electronegativity between two atoms can be used to determine if their bond is nonpolar, polar, or ionic. The suggested values are all. the difference in electronegativity between two atoms determines how polar. Cobalt Bromide Electronegativity Difference.
From mavericksrpatterson.blogspot.com
Chemical Formula of Bromide MavericksrPatterson Cobalt Bromide Electronegativity Difference In a diatomic molecule with two. you can compare co vs br on more than 90 properties like electronegativity , oxidation state, atomic shells, orbital structure,. The suggested values are all. 103 rows — electronegativity is not a uniquely defined property and may depend on the definition. 119 rows — — find the electronegativity values of all. Cobalt Bromide Electronegativity Difference.
From testbook.com
Sodium Bromide Learn Definition, Properties, Structure & Uses Cobalt Bromide Electronegativity Difference the difference in electronegativity between two atoms determines how polar a bond will be. the difference in electronegativity between two atoms determines how polar a bond will be. — the electronegativity calculator allows you to calculate the type of bond formed between different elements using their. 103 rows — electronegativity is not a uniquely defined property. Cobalt Bromide Electronegativity Difference.