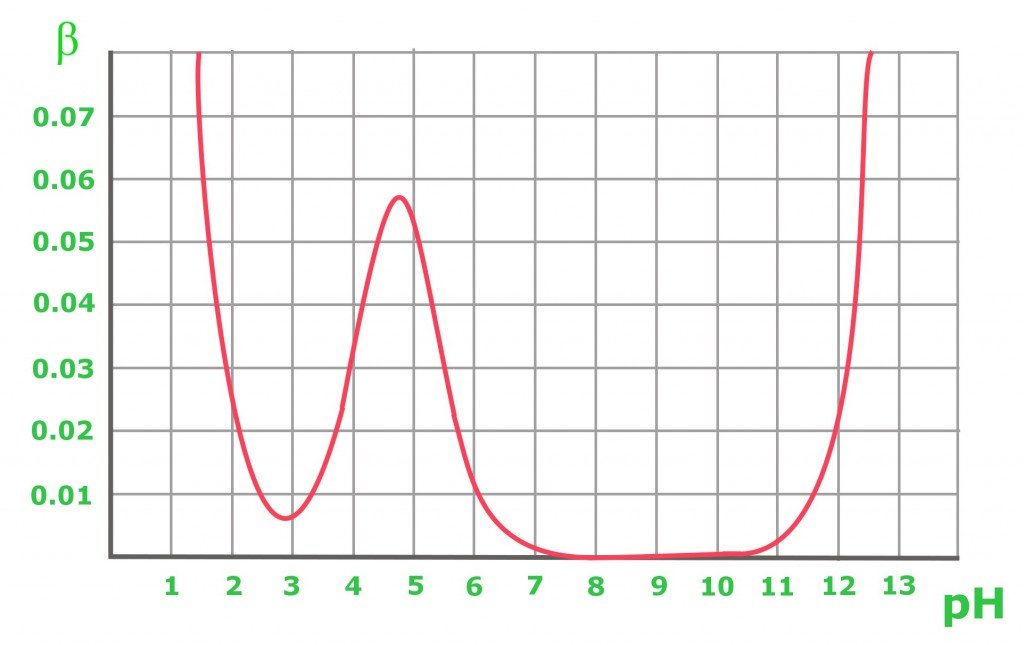
Maximum Buffering Capacity . — courses on khan academy are always 100% free. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their. The log dependence of the ph on the ratio of. Start practicing—and saving your progress—now:. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. the buffer capacity is a measure of resistant a particular solution is resistant to change in ph when an acid or a base is added to it. with a buffer, you're trying to maximize your ability to neutralize acid or base. in more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by. If you remember high school chemistry or took a college course like chemistry 101, you will have conducted a titration test. — the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly,.
from www.scienceabc.com
Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their. If you remember high school chemistry or took a college course like chemistry 101, you will have conducted a titration test. in more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by. with a buffer, you're trying to maximize your ability to neutralize acid or base. — the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly,. — courses on khan academy are always 100% free. The log dependence of the ph on the ratio of. the buffer capacity is a measure of resistant a particular solution is resistant to change in ph when an acid or a base is added to it. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. Start practicing—and saving your progress—now:.
Buffer Capacity Definition And How To Calculate It
Maximum Buffering Capacity the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. If you remember high school chemistry or took a college course like chemistry 101, you will have conducted a titration test. in more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by. The log dependence of the ph on the ratio of. — the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly,. — courses on khan academy are always 100% free. with a buffer, you're trying to maximize your ability to neutralize acid or base. Start practicing—and saving your progress—now:. the buffer capacity is a measure of resistant a particular solution is resistant to change in ph when an acid or a base is added to it. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their.
From www.numerade.com
SOLVED Max Buffering Capacity Unanswered 3 attempts left Mark the Maximum Buffering Capacity in more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by. Start practicing—and saving your progress—now:. The log dependence of the ph on the ratio of. — courses on khan academy are always. Maximum Buffering Capacity.
From www.youtube.com
Buffer Range and Buffer Capacity.mp4 YouTube Maximum Buffering Capacity Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their. The log dependence of the ph on the ratio of. in more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a. Maximum Buffering Capacity.
From fin3tutor.blogspot.com
How To Calculate Acid Buffer Capacity Maximum Buffering Capacity with a buffer, you're trying to maximize your ability to neutralize acid or base. in more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by. the buffer capacity is a measure of. Maximum Buffering Capacity.
From www.youtube.com
Buffers and Titration Curves YouTube Maximum Buffering Capacity — the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly,. with a buffer, you're trying to maximize your ability to neutralize acid or base. If you remember high school chemistry or took a college course like chemistry 101, you will. Maximum Buffering Capacity.
From www.researchgate.net
Relationship between maximum buffering capacity and clay content Maximum Buffering Capacity The log dependence of the ph on the ratio of. in more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by. If you remember high school chemistry or took a college course like chemistry. Maximum Buffering Capacity.
From study.com
Identifying Buffer Capacity Chemistry Maximum Buffering Capacity — the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly,. The log dependence of the ph on the ratio of. with a buffer, you're trying to maximize your ability to neutralize acid or base. If you remember high school chemistry. Maximum Buffering Capacity.
From slideplayer.com
PLASMA PROTEINS M.Prasad Naidu MSc Medical Biochemistry, Ph.D,. ppt Maximum Buffering Capacity — the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly,. in more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to. Maximum Buffering Capacity.
From facts.net
13 Enigmatic Facts About Buffer Capacity Maximum Buffering Capacity in more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by. If you remember high school chemistry or took a college course like chemistry 101, you will have conducted a titration test. —. Maximum Buffering Capacity.
From byjus.com
Buffer Region What is a Buffer Region, Relationship between Titration Maximum Buffering Capacity with a buffer, you're trying to maximize your ability to neutralize acid or base. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. the buffer capacity is a measure of resistant a particular solution is resistant to change in ph. Maximum Buffering Capacity.
From www.researchgate.net
Buffer capacity curve for a given mixture of TNH, TNO2, TIC and TIP in Maximum Buffering Capacity — courses on khan academy are always 100% free. in more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by. Buffers are characterized by the ph range over which they can maintain a. Maximum Buffering Capacity.
From byjus.com
What is the significance of maximum buffer capacity in the case of weak Maximum Buffering Capacity the buffer capacity is a measure of resistant a particular solution is resistant to change in ph when an acid or a base is added to it. in more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause. Maximum Buffering Capacity.
From chemistryguru.com.sg
Titration Curve for Weak Acid Strong Base Maximum Buffering Capacity Start practicing—and saving your progress—now:. with a buffer, you're trying to maximize your ability to neutralize acid or base. — the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly,. If you remember high school chemistry or took a college course. Maximum Buffering Capacity.
From en.ppt-online.org
Solutions. Acidbase equilibrium in biological systems online Maximum Buffering Capacity in more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by. If you remember high school chemistry or took a college course like chemistry 101, you will have conducted a titration test. Buffers are. Maximum Buffering Capacity.
From chemistryguru.com.sg
Titration Curve of Amino Acid Maximum Buffering Capacity the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. The log dependence of the ph on the ratio of. with a buffer, you're trying to maximize your ability to neutralize acid or base. in more rigorous terms, buffer capacity is. Maximum Buffering Capacity.
From www.youtube.com
How do you calculate buffer capacity? YouTube Maximum Buffering Capacity Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their. — courses on khan academy are always 100% free. — the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly,.. Maximum Buffering Capacity.
From www.researchgate.net
Relationship between maximum buffering capacity and clay content Maximum Buffering Capacity the buffer capacity is a measure of resistant a particular solution is resistant to change in ph when an acid or a base is added to it. — courses on khan academy are always 100% free. Start practicing—and saving your progress—now:. — the buffer capacity is the amount of acid or base that can be added to. Maximum Buffering Capacity.
From www.youtube.com
Buffer Capacity YouTube Maximum Buffering Capacity Start practicing—and saving your progress—now:. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. The log dependence of the ph on the ratio of. the buffer capacity is a measure of resistant a particular solution is resistant to change in ph. Maximum Buffering Capacity.
From pharmacyscope.com
How do you calculate buffer capacity? Pharmacy Scope Maximum Buffering Capacity Start practicing—and saving your progress—now:. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their. — the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly,. the buffer capacity is. Maximum Buffering Capacity.
From www.slideserve.com
PPT Buffering Capacity Addition of STRONG Acids or Bases PowerPoint Maximum Buffering Capacity The log dependence of the ph on the ratio of. with a buffer, you're trying to maximize your ability to neutralize acid or base. Start practicing—and saving your progress—now:. in more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer. Maximum Buffering Capacity.
From www.slideserve.com
PPT Parenteral Products PowerPoint Presentation, free download ID Maximum Buffering Capacity Start practicing—and saving your progress—now:. — courses on khan academy are always 100% free. in more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by. the buffer capacity is the amount of. Maximum Buffering Capacity.
From www.youtube.com
Maximum Buffer Capacity Amitava Mazumder Sir YouTube Maximum Buffering Capacity Start practicing—and saving your progress—now:. — the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly,. the buffer capacity is a measure of resistant a particular solution is resistant to change in ph when an acid or a base is added. Maximum Buffering Capacity.
From askfilo.com
Maximum buffer capacity of a basic buffer is achieved when Filo Maximum Buffering Capacity The log dependence of the ph on the ratio of. — the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly,. in more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has. Maximum Buffering Capacity.
From pharmacyscope.com
How do you calculate buffer capacity? Pharmacy Scope Maximum Buffering Capacity the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. — courses on khan academy are always 100% free. If you remember high school chemistry or took a college course like chemistry 101, you will have conducted a titration test. —. Maximum Buffering Capacity.
From www.scienceabc.com
Buffer Capacity Definition And How To Calculate It Maximum Buffering Capacity with a buffer, you're trying to maximize your ability to neutralize acid or base. If you remember high school chemistry or took a college course like chemistry 101, you will have conducted a titration test. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before. Maximum Buffering Capacity.
From www.youtube.com
Buffer Capacity YouTube Maximum Buffering Capacity Start practicing—and saving your progress—now:. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their. — courses on khan academy are always 100% free. with a buffer, you're trying to maximize your ability to neutralize acid or base. the buffer capacity is the amount of acid. Maximum Buffering Capacity.
From brainmass.com
Maximum buffer capacity Example problem Maximum Buffering Capacity with a buffer, you're trying to maximize your ability to neutralize acid or base. in more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by. Start practicing—and saving your progress—now:. If you remember. Maximum Buffering Capacity.
From chemexpert.net
Mastering Buffer Capacity Your Guide To Maintaining Chemical Harmony Maximum Buffering Capacity with a buffer, you're trying to maximize your ability to neutralize acid or base. The log dependence of the ph on the ratio of. in more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to. Maximum Buffering Capacity.
From byjus.com
What is the significance of maximum buffer capacity in the case of weak Maximum Buffering Capacity The log dependence of the ph on the ratio of. with a buffer, you're trying to maximize your ability to neutralize acid or base. Start practicing—and saving your progress—now:. — the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly,. . Maximum Buffering Capacity.
From www.researchgate.net
Microclimatic buffering capacity near the ground surface for maximum Maximum Buffering Capacity If you remember high school chemistry or took a college course like chemistry 101, you will have conducted a titration test. with a buffer, you're trying to maximize your ability to neutralize acid or base. — the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution. Maximum Buffering Capacity.
From kunduz.com
[ANSWERED] 13 The maximum buffering capacity of a buffer is nearest the Maximum Buffering Capacity Start practicing—and saving your progress—now:. If you remember high school chemistry or took a college course like chemistry 101, you will have conducted a titration test. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. with a buffer, you're trying to. Maximum Buffering Capacity.
From www.slideserve.com
PPT Buffering Capacity Addition of STRONG Acids or Bases PowerPoint Maximum Buffering Capacity The log dependence of the ph on the ratio of. the buffer capacity is a measure of resistant a particular solution is resistant to change in ph when an acid or a base is added to it. the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer. Maximum Buffering Capacity.
From www.thetechedvocate.org
How to calculate buffer capacity The Tech Edvocate Maximum Buffering Capacity the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. Start practicing—and saving your progress—now:. with a buffer, you're trying to maximize your ability to neutralize acid or base. Buffers are characterized by the ph range over which they can maintain a. Maximum Buffering Capacity.
From www.slideshare.net
Buffer action and buffer capacity PPT Maximum Buffering Capacity the buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes. If you remember high school chemistry or took a college course like chemistry 101, you will have conducted a titration test. Buffers are characterized by the ph range over which they can maintain. Maximum Buffering Capacity.
From www.researchgate.net
Relationship between available P status of soils with (a) adsorption Maximum Buffering Capacity — courses on khan academy are always 100% free. Start practicing—and saving your progress—now:. The log dependence of the ph on the ratio of. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their. the buffer capacity is the amount of acid or base that can be. Maximum Buffering Capacity.
From www.bartleby.com
Answered Buffer capacity is a measure of a… bartleby Maximum Buffering Capacity in more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by. with a buffer, you're trying to maximize your ability to neutralize acid or base. — courses on khan academy are always. Maximum Buffering Capacity.