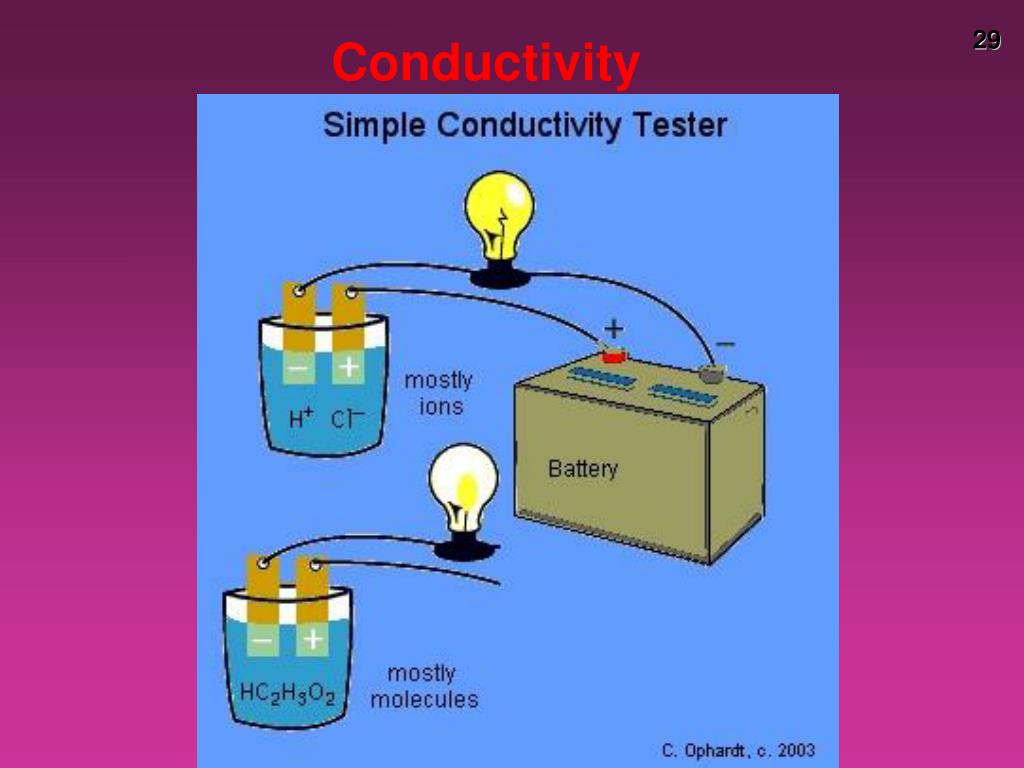
Conductivity Chemistry Example . Conductivity is the transfer of energy through contact with a material or materials. Conductivity is represented by \( \sigma \) and is measured in siemens (\(1/ωm\)). It can refer to electric conductivity, acoustic conductivity or thermal conductivity. Since conductivity is the measure of how easily. Describe and explain the difference in conductivity between strong electrolytes, weak electrolytes,. In this article, we shall discuss electrical conductance and conductivity, its definition, formula, unit of measurement, and. Electrical conductivity is the ability of a material to carry the flow of an electric current (a flow of electrons). To determine of the solution is a strong or weak electrolyte; The si unit of conductivity. To observe electrical conductivity of substances in various aqueous solutions; Imagine that you attach the two ends of a battery to a bar of iron and a. To investigate and explain factors affecting the solubility of different aqueous solutions. Conductivity or specific conductance of an electrolyte solution is a measure of its ability to conduct electricity. To interpret a chemical reaction by observing.
from www.slideserve.com
Conductivity is the transfer of energy through contact with a material or materials. Conductivity or specific conductance of an electrolyte solution is a measure of its ability to conduct electricity. Imagine that you attach the two ends of a battery to a bar of iron and a. Describe and explain the difference in conductivity between strong electrolytes, weak electrolytes,. To observe electrical conductivity of substances in various aqueous solutions; To determine of the solution is a strong or weak electrolyte; To investigate and explain factors affecting the solubility of different aqueous solutions. Conductivity is represented by \( \sigma \) and is measured in siemens (\(1/ωm\)). The si unit of conductivity. To interpret a chemical reaction by observing.
PPT The Chemistry of Acids and Bases PowerPoint Presentation, free
Conductivity Chemistry Example To interpret a chemical reaction by observing. Conductivity is represented by \( \sigma \) and is measured in siemens (\(1/ωm\)). To observe electrical conductivity of substances in various aqueous solutions; Electrical conductivity is the ability of a material to carry the flow of an electric current (a flow of electrons). Imagine that you attach the two ends of a battery to a bar of iron and a. Describe and explain the difference in conductivity between strong electrolytes, weak electrolytes,. Conductivity or specific conductance of an electrolyte solution is a measure of its ability to conduct electricity. To investigate and explain factors affecting the solubility of different aqueous solutions. To interpret a chemical reaction by observing. Since conductivity is the measure of how easily. The si unit of conductivity. Conductivity is the transfer of energy through contact with a material or materials. In this article, we shall discuss electrical conductance and conductivity, its definition, formula, unit of measurement, and. It can refer to electric conductivity, acoustic conductivity or thermal conductivity. To determine of the solution is a strong or weak electrolyte;
From sciencenotes.org
What Is the Most Conductive Element? Conductivity Chemistry Example It can refer to electric conductivity, acoustic conductivity or thermal conductivity. To interpret a chemical reaction by observing. To investigate and explain factors affecting the solubility of different aqueous solutions. Imagine that you attach the two ends of a battery to a bar of iron and a. The si unit of conductivity. Since conductivity is the measure of how easily.. Conductivity Chemistry Example.
From www.thoughtco.com
Conductivity and Conductive Elements Conductivity Chemistry Example In this article, we shall discuss electrical conductance and conductivity, its definition, formula, unit of measurement, and. To observe electrical conductivity of substances in various aqueous solutions; Since conductivity is the measure of how easily. Conductivity is represented by \( \sigma \) and is measured in siemens (\(1/ωm\)). Imagine that you attach the two ends of a battery to a. Conductivity Chemistry Example.
From www.thoughtco.com
Electrical Conductivity of Metals Conductivity Chemistry Example Since conductivity is the measure of how easily. Imagine that you attach the two ends of a battery to a bar of iron and a. In this article, we shall discuss electrical conductance and conductivity, its definition, formula, unit of measurement, and. Conductivity is represented by \( \sigma \) and is measured in siemens (\(1/ωm\)). The si unit of conductivity.. Conductivity Chemistry Example.
From www.youtube.com
Molar Conductivity and Equivalent Conductivity Class 12 Chemistry Conductivity Chemistry Example The si unit of conductivity. Describe and explain the difference in conductivity between strong electrolytes, weak electrolytes,. It can refer to electric conductivity, acoustic conductivity or thermal conductivity. Since conductivity is the measure of how easily. Conductivity is represented by \( \sigma \) and is measured in siemens (\(1/ωm\)). In this article, we shall discuss electrical conductance and conductivity, its. Conductivity Chemistry Example.
From www.slideserve.com
PPT Pure Substances vs. Mixtures Physical and Chemical Changes Conductivity Chemistry Example To investigate and explain factors affecting the solubility of different aqueous solutions. It can refer to electric conductivity, acoustic conductivity or thermal conductivity. Conductivity or specific conductance of an electrolyte solution is a measure of its ability to conduct electricity. Describe and explain the difference in conductivity between strong electrolytes, weak electrolytes,. Conductivity is represented by \( \sigma \) and. Conductivity Chemistry Example.
From www.animalia-life.club
Conductivity Chemistry Lab Conductivity Chemistry Example To determine of the solution is a strong or weak electrolyte; In this article, we shall discuss electrical conductance and conductivity, its definition, formula, unit of measurement, and. Electrical conductivity is the ability of a material to carry the flow of an electric current (a flow of electrons). To investigate and explain factors affecting the solubility of different aqueous solutions.. Conductivity Chemistry Example.
From www.youtube.com
specific conductance or conductivity electro chemistry chapter 3 Conductivity Chemistry Example In this article, we shall discuss electrical conductance and conductivity, its definition, formula, unit of measurement, and. Imagine that you attach the two ends of a battery to a bar of iron and a. To determine of the solution is a strong or weak electrolyte; Electrical conductivity is the ability of a material to carry the flow of an electric. Conductivity Chemistry Example.
From www.slideserve.com
PPT ELECTROLYTE CONDUCTANCE PowerPoint Presentation, free download Conductivity Chemistry Example To determine of the solution is a strong or weak electrolyte; Since conductivity is the measure of how easily. Describe and explain the difference in conductivity between strong electrolytes, weak electrolytes,. To interpret a chemical reaction by observing. Conductivity is the transfer of energy through contact with a material or materials. The si unit of conductivity. In this article, we. Conductivity Chemistry Example.
From www.slideserve.com
PPT The Chemistry of Acids and Bases PowerPoint Presentation, free Conductivity Chemistry Example To investigate and explain factors affecting the solubility of different aqueous solutions. Conductivity is represented by \( \sigma \) and is measured in siemens (\(1/ωm\)). Since conductivity is the measure of how easily. To determine of the solution is a strong or weak electrolyte; Imagine that you attach the two ends of a battery to a bar of iron and. Conductivity Chemistry Example.
From www.youtube.com
Problem 1 on molar conductivity (Electrochemistry part 54 for CBSE Conductivity Chemistry Example Since conductivity is the measure of how easily. In this article, we shall discuss electrical conductance and conductivity, its definition, formula, unit of measurement, and. Conductivity is the transfer of energy through contact with a material or materials. To interpret a chemical reaction by observing. Conductivity or specific conductance of an electrolyte solution is a measure of its ability to. Conductivity Chemistry Example.
From classnotes.org.in
Variation of Conductivity and Molar Conductivity Chemistry, Class 12 Conductivity Chemistry Example Conductivity or specific conductance of an electrolyte solution is a measure of its ability to conduct electricity. The si unit of conductivity. To interpret a chemical reaction by observing. Conductivity is represented by \( \sigma \) and is measured in siemens (\(1/ωm\)). To investigate and explain factors affecting the solubility of different aqueous solutions. To determine of the solution is. Conductivity Chemistry Example.
From www.youtube.com
equivalent and molar conductance electrical conductance quantities Conductivity Chemistry Example To interpret a chemical reaction by observing. It can refer to electric conductivity, acoustic conductivity or thermal conductivity. To investigate and explain factors affecting the solubility of different aqueous solutions. Conductivity is the transfer of energy through contact with a material or materials. Conductivity is represented by \( \sigma \) and is measured in siemens (\(1/ωm\)). Since conductivity is the. Conductivity Chemistry Example.
From www.slideserve.com
PPT Conducting Polymers PowerPoint Presentation ID403789 Conductivity Chemistry Example To investigate and explain factors affecting the solubility of different aqueous solutions. Describe and explain the difference in conductivity between strong electrolytes, weak electrolytes,. Since conductivity is the measure of how easily. Imagine that you attach the two ends of a battery to a bar of iron and a. Conductivity is represented by \( \sigma \) and is measured in. Conductivity Chemistry Example.
From www.reagent.co.uk
What Is Conductivity In Chemistry? The Science Blog Conductivity Chemistry Example In this article, we shall discuss electrical conductance and conductivity, its definition, formula, unit of measurement, and. The si unit of conductivity. Conductivity or specific conductance of an electrolyte solution is a measure of its ability to conduct electricity. Describe and explain the difference in conductivity between strong electrolytes, weak electrolytes,. To determine of the solution is a strong or. Conductivity Chemistry Example.
From www.youtube.com
Chemistry Liquid Conductivity, Electrolysis and simple Voltaic cell Conductivity Chemistry Example Describe and explain the difference in conductivity between strong electrolytes, weak electrolytes,. Since conductivity is the measure of how easily. Conductivity is represented by \( \sigma \) and is measured in siemens (\(1/ωm\)). Electrical conductivity is the ability of a material to carry the flow of an electric current (a flow of electrons). To investigate and explain factors affecting the. Conductivity Chemistry Example.
From www.youtube.com
Variation of Conductivity and Molar Conductivity with Concentration Conductivity Chemistry Example Conductivity is represented by \( \sigma \) and is measured in siemens (\(1/ωm\)). To interpret a chemical reaction by observing. To observe electrical conductivity of substances in various aqueous solutions; Electrical conductivity is the ability of a material to carry the flow of an electric current (a flow of electrons). Since conductivity is the measure of how easily. Conductivity or. Conductivity Chemistry Example.
From fphoto.photoshelter.com
science chemistry conductivity test Fundamental Photographs The Art Conductivity Chemistry Example Conductivity or specific conductance of an electrolyte solution is a measure of its ability to conduct electricity. Since conductivity is the measure of how easily. Electrical conductivity is the ability of a material to carry the flow of an electric current (a flow of electrons). To interpret a chemical reaction by observing. Describe and explain the difference in conductivity between. Conductivity Chemistry Example.
From www.slideserve.com
PPT Properties of Substances PowerPoint Presentation, free download Conductivity Chemistry Example Conductivity is the transfer of energy through contact with a material or materials. Imagine that you attach the two ends of a battery to a bar of iron and a. Electrical conductivity is the ability of a material to carry the flow of an electric current (a flow of electrons). Describe and explain the difference in conductivity between strong electrolytes,. Conductivity Chemistry Example.
From keegan-has-dodson.blogspot.com
Conductivity Is Best Described as the Ability of KeeganhasDodson Conductivity Chemistry Example To determine of the solution is a strong or weak electrolyte; To observe electrical conductivity of substances in various aqueous solutions; Since conductivity is the measure of how easily. Electrical conductivity is the ability of a material to carry the flow of an electric current (a flow of electrons). Imagine that you attach the two ends of a battery to. Conductivity Chemistry Example.
From examples.yourdictionary.com
Examples of Conduction Main Types YourDictionary Conductivity Chemistry Example Imagine that you attach the two ends of a battery to a bar of iron and a. To determine of the solution is a strong or weak electrolyte; To investigate and explain factors affecting the solubility of different aqueous solutions. To observe electrical conductivity of substances in various aqueous solutions; It can refer to electric conductivity, acoustic conductivity or thermal. Conductivity Chemistry Example.
From sciencenotes.org
What Is the Most Conductive Element? Conductivity Chemistry Example Conductivity is represented by \( \sigma \) and is measured in siemens (\(1/ωm\)). Conductivity or specific conductance of an electrolyte solution is a measure of its ability to conduct electricity. To observe electrical conductivity of substances in various aqueous solutions; Describe and explain the difference in conductivity between strong electrolytes, weak electrolytes,. To investigate and explain factors affecting the solubility. Conductivity Chemistry Example.
From atlas-scientific.com
How Do Ions Increase Conductivity? Atlas Scientific Conductivity Chemistry Example It can refer to electric conductivity, acoustic conductivity or thermal conductivity. The si unit of conductivity. Conductivity is the transfer of energy through contact with a material or materials. Since conductivity is the measure of how easily. Conductivity is represented by \( \sigma \) and is measured in siemens (\(1/ωm\)). Conductivity or specific conductance of an electrolyte solution is a. Conductivity Chemistry Example.
From www.reagent.co.uk
What Is Conductivity In Chemistry? The Science Blog Conductivity Chemistry Example Since conductivity is the measure of how easily. Imagine that you attach the two ends of a battery to a bar of iron and a. To interpret a chemical reaction by observing. To investigate and explain factors affecting the solubility of different aqueous solutions. The si unit of conductivity. It can refer to electric conductivity, acoustic conductivity or thermal conductivity.. Conductivity Chemistry Example.
From group.chem.iastate.edu
Conductivity Meter Holme Research Group Iowa State University Conductivity Chemistry Example It can refer to electric conductivity, acoustic conductivity or thermal conductivity. Conductivity is the transfer of energy through contact with a material or materials. To determine of the solution is a strong or weak electrolyte; Electrical conductivity is the ability of a material to carry the flow of an electric current (a flow of electrons). To observe electrical conductivity of. Conductivity Chemistry Example.
From www.youtube.com
How To calculate Conductivity using EIS YouTube Conductivity Chemistry Example To determine of the solution is a strong or weak electrolyte; To investigate and explain factors affecting the solubility of different aqueous solutions. In this article, we shall discuss electrical conductance and conductivity, its definition, formula, unit of measurement, and. Conductivity is represented by \( \sigma \) and is measured in siemens (\(1/ωm\)). The si unit of conductivity. Conductivity is. Conductivity Chemistry Example.
From fphoto.photoshelter.com
science chemistry conductivity test Fundamental Photographs The Art Conductivity Chemistry Example To observe electrical conductivity of substances in various aqueous solutions; It can refer to electric conductivity, acoustic conductivity or thermal conductivity. The si unit of conductivity. In this article, we shall discuss electrical conductance and conductivity, its definition, formula, unit of measurement, and. To determine of the solution is a strong or weak electrolyte; To investigate and explain factors affecting. Conductivity Chemistry Example.
From www.youtube.com
Conductivity Basics YouTube Conductivity Chemistry Example It can refer to electric conductivity, acoustic conductivity or thermal conductivity. In this article, we shall discuss electrical conductance and conductivity, its definition, formula, unit of measurement, and. Electrical conductivity is the ability of a material to carry the flow of an electric current (a flow of electrons). Conductivity is the transfer of energy through contact with a material or. Conductivity Chemistry Example.
From pilgaard.info
Conductivity Ions in solution Michael Pilgaard's Chemistry Conductivity Chemistry Example The si unit of conductivity. Conductivity or specific conductance of an electrolyte solution is a measure of its ability to conduct electricity. In this article, we shall discuss electrical conductance and conductivity, its definition, formula, unit of measurement, and. Electrical conductivity is the ability of a material to carry the flow of an electric current (a flow of electrons). To. Conductivity Chemistry Example.
From youtube.com
Electrochemistry History and nomenclature, Conductivity, Conductance Conductivity Chemistry Example To observe electrical conductivity of substances in various aqueous solutions; In this article, we shall discuss electrical conductance and conductivity, its definition, formula, unit of measurement, and. Electrical conductivity is the ability of a material to carry the flow of an electric current (a flow of electrons). It can refer to electric conductivity, acoustic conductivity or thermal conductivity. To investigate. Conductivity Chemistry Example.
From www.youtube.com
Conductivity of Solutions Demonstration YouTube Conductivity Chemistry Example Conductivity or specific conductance of an electrolyte solution is a measure of its ability to conduct electricity. It can refer to electric conductivity, acoustic conductivity or thermal conductivity. To determine of the solution is a strong or weak electrolyte; Conductivity is the transfer of energy through contact with a material or materials. To investigate and explain factors affecting the solubility. Conductivity Chemistry Example.
From www.slideserve.com
PPT Karst Chemistry II PowerPoint Presentation, free download ID Conductivity Chemistry Example It can refer to electric conductivity, acoustic conductivity or thermal conductivity. Since conductivity is the measure of how easily. In this article, we shall discuss electrical conductance and conductivity, its definition, formula, unit of measurement, and. Conductivity is represented by \( \sigma \) and is measured in siemens (\(1/ωm\)). The si unit of conductivity. Describe and explain the difference in. Conductivity Chemistry Example.
From studylib.net
Lab Conductivity of Ionic and Covalent Compounds Conductivity Chemistry Example Electrical conductivity is the ability of a material to carry the flow of an electric current (a flow of electrons). Conductivity or specific conductance of an electrolyte solution is a measure of its ability to conduct electricity. Imagine that you attach the two ends of a battery to a bar of iron and a. Conductivity is the transfer of energy. Conductivity Chemistry Example.
From www.ck12.org
Physical Properties of Ionic Compounds ( Read ) Chemistry CK12 Conductivity Chemistry Example To investigate and explain factors affecting the solubility of different aqueous solutions. Describe and explain the difference in conductivity between strong electrolytes, weak electrolytes,. Electrical conductivity is the ability of a material to carry the flow of an electric current (a flow of electrons). It can refer to electric conductivity, acoustic conductivity or thermal conductivity. The si unit of conductivity.. Conductivity Chemistry Example.
From www.slideserve.com
PPT ELECTROLYTE CONDUCTANCE PowerPoint Presentation, free download Conductivity Chemistry Example It can refer to electric conductivity, acoustic conductivity or thermal conductivity. To determine of the solution is a strong or weak electrolyte; Imagine that you attach the two ends of a battery to a bar of iron and a. Conductivity is represented by \( \sigma \) and is measured in siemens (\(1/ωm\)). In this article, we shall discuss electrical conductance. Conductivity Chemistry Example.
From fphoto.photoshelter.com
chemistry conductivity test Fundamental Photographs The Art of Science Conductivity Chemistry Example To observe electrical conductivity of substances in various aqueous solutions; Conductivity is the transfer of energy through contact with a material or materials. Electrical conductivity is the ability of a material to carry the flow of an electric current (a flow of electrons). Conductivity or specific conductance of an electrolyte solution is a measure of its ability to conduct electricity.. Conductivity Chemistry Example.