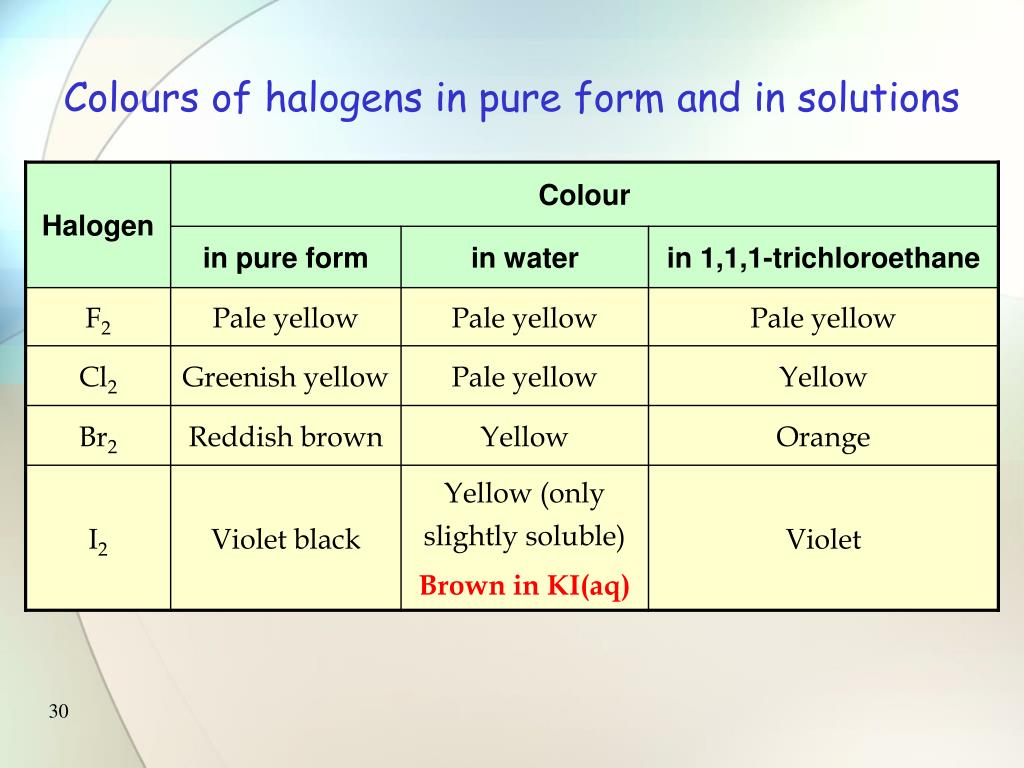
Acidic Property Of Halogens In Water . It is a regular tetrahedral molecule, and reacts violently with water forming silicic acid and hydrochloric acid. The electronegativity of an element is a measurement of the strength of its atom in a molecule to pull electrons towards its nucleus. It is useful as a raw material for the production of pure silicon,. All of the halogens occur in seawater as halide ions. Halogens react to a small extent with water, forming acidic solutions with bleaching properties. The concentration of the chloride ion is 0.54 m; This page discusses the acidity of the hydrogen halides: Hydrohalic acids are commonly termed hydrogen halides when they dissolve in water to give acids. They also undergo redox reactions with metal halides in solution, displacing. Chlorine, bromine and iodine are soluble in water to form an acidic solution. The solubility decreases down the group. Hydrogen fluoride, hydrogen chloride, hydrogen bromide and hydrogen. Hydrochloric acid, sometimes called muriatic acid, is a commonly used acid in industry and laboratories.
from www.slideserve.com
The concentration of the chloride ion is 0.54 m; Hydrogen fluoride, hydrogen chloride, hydrogen bromide and hydrogen. The electronegativity of an element is a measurement of the strength of its atom in a molecule to pull electrons towards its nucleus. It is a regular tetrahedral molecule, and reacts violently with water forming silicic acid and hydrochloric acid. They also undergo redox reactions with metal halides in solution, displacing. Hydrochloric acid, sometimes called muriatic acid, is a commonly used acid in industry and laboratories. The solubility decreases down the group. This page discusses the acidity of the hydrogen halides: Halogens react to a small extent with water, forming acidic solutions with bleaching properties. Hydrohalic acids are commonly termed hydrogen halides when they dissolve in water to give acids.
PPT Characteristic Properties of the Halogens PowerPoint Presentation ID370141
Acidic Property Of Halogens In Water The solubility decreases down the group. They also undergo redox reactions with metal halides in solution, displacing. Halogens react to a small extent with water, forming acidic solutions with bleaching properties. The solubility decreases down the group. Hydrohalic acids are commonly termed hydrogen halides when they dissolve in water to give acids. It is a regular tetrahedral molecule, and reacts violently with water forming silicic acid and hydrochloric acid. It is useful as a raw material for the production of pure silicon,. Hydrochloric acid, sometimes called muriatic acid, is a commonly used acid in industry and laboratories. The concentration of the chloride ion is 0.54 m; All of the halogens occur in seawater as halide ions. The electronegativity of an element is a measurement of the strength of its atom in a molecule to pull electrons towards its nucleus. This page discusses the acidity of the hydrogen halides: Chlorine, bromine and iodine are soluble in water to form an acidic solution. Hydrogen fluoride, hydrogen chloride, hydrogen bromide and hydrogen.
From www.youtube.com
Physical properties of halogens YouTube Acidic Property Of Halogens In Water This page discusses the acidity of the hydrogen halides: The electronegativity of an element is a measurement of the strength of its atom in a molecule to pull electrons towards its nucleus. The concentration of the chloride ion is 0.54 m; Hydrochloric acid, sometimes called muriatic acid, is a commonly used acid in industry and laboratories. The solubility decreases down. Acidic Property Of Halogens In Water.
From www.pinterest.com
Pin on Periodic Table Halogen Acidic Property Of Halogens In Water It is a regular tetrahedral molecule, and reacts violently with water forming silicic acid and hydrochloric acid. The solubility decreases down the group. All of the halogens occur in seawater as halide ions. Hydrohalic acids are commonly termed hydrogen halides when they dissolve in water to give acids. Chlorine, bromine and iodine are soluble in water to form an acidic. Acidic Property Of Halogens In Water.
From www.slideserve.com
PPT Group 7 the halogens PowerPoint Presentation, free download ID3005113 Acidic Property Of Halogens In Water It is useful as a raw material for the production of pure silicon,. Chlorine, bromine and iodine are soluble in water to form an acidic solution. It is a regular tetrahedral molecule, and reacts violently with water forming silicic acid and hydrochloric acid. All of the halogens occur in seawater as halide ions. Hydrogen fluoride, hydrogen chloride, hydrogen bromide and. Acidic Property Of Halogens In Water.
From www.doubtnut.com
Doubt Solutions Maths, Science, CBSE, NCERT, IIT JEE, NEET Acidic Property Of Halogens In Water Hydrohalic acids are commonly termed hydrogen halides when they dissolve in water to give acids. It is useful as a raw material for the production of pure silicon,. Hydrochloric acid, sometimes called muriatic acid, is a commonly used acid in industry and laboratories. All of the halogens occur in seawater as halide ions. The solubility decreases down the group. Chlorine,. Acidic Property Of Halogens In Water.
From ppt-online.org
Study of the properties of halogens and the determination of halide ions in aqueous solution Acidic Property Of Halogens In Water Hydrogen fluoride, hydrogen chloride, hydrogen bromide and hydrogen. Halogens react to a small extent with water, forming acidic solutions with bleaching properties. The electronegativity of an element is a measurement of the strength of its atom in a molecule to pull electrons towards its nucleus. All of the halogens occur in seawater as halide ions. They also undergo redox reactions. Acidic Property Of Halogens In Water.
From www.slideserve.com
PPT The Halogens PowerPoint Presentation, free download ID5949337 Acidic Property Of Halogens In Water Hydrochloric acid, sometimes called muriatic acid, is a commonly used acid in industry and laboratories. Halogens react to a small extent with water, forming acidic solutions with bleaching properties. Hydrohalic acids are commonly termed hydrogen halides when they dissolve in water to give acids. All of the halogens occur in seawater as halide ions. The electronegativity of an element is. Acidic Property Of Halogens In Water.
From spmchemistry.blog.onlinetuition.com.my
Role of Water to Show Properties of Acids SPM Chemistry Acidic Property Of Halogens In Water Chlorine, bromine and iodine are soluble in water to form an acidic solution. All of the halogens occur in seawater as halide ions. Hydrochloric acid, sometimes called muriatic acid, is a commonly used acid in industry and laboratories. It is useful as a raw material for the production of pure silicon,. This page discusses the acidity of the hydrogen halides:. Acidic Property Of Halogens In Water.
From www.slideserve.com
PPT Halogens PowerPoint Presentation, free download ID6732639 Acidic Property Of Halogens In Water Hydrohalic acids are commonly termed hydrogen halides when they dissolve in water to give acids. Hydrogen fluoride, hydrogen chloride, hydrogen bromide and hydrogen. The concentration of the chloride ion is 0.54 m; It is useful as a raw material for the production of pure silicon,. Hydrochloric acid, sometimes called muriatic acid, is a commonly used acid in industry and laboratories.. Acidic Property Of Halogens In Water.
From www.slideserve.com
PPT The Halogens PowerPoint Presentation, free download ID2972542 Acidic Property Of Halogens In Water This page discusses the acidity of the hydrogen halides: It is a regular tetrahedral molecule, and reacts violently with water forming silicic acid and hydrochloric acid. The electronegativity of an element is a measurement of the strength of its atom in a molecule to pull electrons towards its nucleus. Chlorine, bromine and iodine are soluble in water to form an. Acidic Property Of Halogens In Water.
From www.slideserve.com
PPT Group 7 the halogens PowerPoint Presentation, free download ID3005113 Acidic Property Of Halogens In Water Chlorine, bromine and iodine are soluble in water to form an acidic solution. The electronegativity of an element is a measurement of the strength of its atom in a molecule to pull electrons towards its nucleus. Hydrochloric acid, sometimes called muriatic acid, is a commonly used acid in industry and laboratories. It is useful as a raw material for the. Acidic Property Of Halogens In Water.
From www.slideserve.com
PPT Characteristic Properties of the Halogens PowerPoint Presentation ID370141 Acidic Property Of Halogens In Water The electronegativity of an element is a measurement of the strength of its atom in a molecule to pull electrons towards its nucleus. Chlorine, bromine and iodine are soluble in water to form an acidic solution. Hydrohalic acids are commonly termed hydrogen halides when they dissolve in water to give acids. It is useful as a raw material for the. Acidic Property Of Halogens In Water.
From www.slideserve.com
PPT Halogens PowerPoint Presentation, free download ID6702617 Acidic Property Of Halogens In Water Hydrogen fluoride, hydrogen chloride, hydrogen bromide and hydrogen. The solubility decreases down the group. They also undergo redox reactions with metal halides in solution, displacing. All of the halogens occur in seawater as halide ions. Chlorine, bromine and iodine are soluble in water to form an acidic solution. Hydrohalic acids are commonly termed hydrogen halides when they dissolve in water. Acidic Property Of Halogens In Water.
From slideplayer.com
The Halogens F Cl Br I At. ppt download Acidic Property Of Halogens In Water The solubility decreases down the group. It is useful as a raw material for the production of pure silicon,. All of the halogens occur in seawater as halide ions. Hydrogen fluoride, hydrogen chloride, hydrogen bromide and hydrogen. This page discusses the acidity of the hydrogen halides: Chlorine, bromine and iodine are soluble in water to form an acidic solution. Halogens. Acidic Property Of Halogens In Water.
From www.slideserve.com
PPT Physical properties of halogens PowerPoint Presentation, free download ID5730329 Acidic Property Of Halogens In Water Hydrogen fluoride, hydrogen chloride, hydrogen bromide and hydrogen. It is a regular tetrahedral molecule, and reacts violently with water forming silicic acid and hydrochloric acid. The electronegativity of an element is a measurement of the strength of its atom in a molecule to pull electrons towards its nucleus. Chlorine, bromine and iodine are soluble in water to form an acidic. Acidic Property Of Halogens In Water.
From www.slideserve.com
PPT Group 7 The Halogens PowerPoint Presentation, free download ID1275374 Acidic Property Of Halogens In Water Chlorine, bromine and iodine are soluble in water to form an acidic solution. All of the halogens occur in seawater as halide ions. The solubility decreases down the group. Halogens react to a small extent with water, forming acidic solutions with bleaching properties. It is a regular tetrahedral molecule, and reacts violently with water forming silicic acid and hydrochloric acid.. Acidic Property Of Halogens In Water.
From www.youtube.com
Chemical properties of Group13 elements ( Reaction with Oxygen,Acids & Alkalis,Halogen,Water Acidic Property Of Halogens In Water The solubility decreases down the group. Hydrogen fluoride, hydrogen chloride, hydrogen bromide and hydrogen. The concentration of the chloride ion is 0.54 m; It is useful as a raw material for the production of pure silicon,. Hydrohalic acids are commonly termed hydrogen halides when they dissolve in water to give acids. All of the halogens occur in seawater as halide. Acidic Property Of Halogens In Water.
From collegedunia.com
Halogens Properties, Electronic Configuration & Characteristics Acidic Property Of Halogens In Water This page discusses the acidity of the hydrogen halides: The concentration of the chloride ion is 0.54 m; All of the halogens occur in seawater as halide ions. The solubility decreases down the group. Hydrochloric acid, sometimes called muriatic acid, is a commonly used acid in industry and laboratories. The electronegativity of an element is a measurement of the strength. Acidic Property Of Halogens In Water.
From www.slideserve.com
PPT Chapter 22 Chemistry of the Nonmetals PowerPoint Presentation, free download ID6088597 Acidic Property Of Halogens In Water The solubility decreases down the group. All of the halogens occur in seawater as halide ions. The concentration of the chloride ion is 0.54 m; This page discusses the acidity of the hydrogen halides: They also undergo redox reactions with metal halides in solution, displacing. Hydrohalic acids are commonly termed hydrogen halides when they dissolve in water to give acids.. Acidic Property Of Halogens In Water.
From www.slideserve.com
PPT Group 7 the halogens PowerPoint Presentation, free download ID3005113 Acidic Property Of Halogens In Water The electronegativity of an element is a measurement of the strength of its atom in a molecule to pull electrons towards its nucleus. Halogens react to a small extent with water, forming acidic solutions with bleaching properties. Hydrohalic acids are commonly termed hydrogen halides when they dissolve in water to give acids. They also undergo redox reactions with metal halides. Acidic Property Of Halogens In Water.
From www.slideserve.com
PPT Reactions of the halogens and halide ions PowerPoint Presentation ID2518013 Acidic Property Of Halogens In Water The concentration of the chloride ion is 0.54 m; The solubility decreases down the group. Hydrohalic acids are commonly termed hydrogen halides when they dissolve in water to give acids. They also undergo redox reactions with metal halides in solution, displacing. Hydrogen fluoride, hydrogen chloride, hydrogen bromide and hydrogen. This page discusses the acidity of the hydrogen halides: Hydrochloric acid,. Acidic Property Of Halogens In Water.
From www.youtube.com
Halogens Chemical Properties (Part 2 ) YouTube Acidic Property Of Halogens In Water The solubility decreases down the group. Hydrogen fluoride, hydrogen chloride, hydrogen bromide and hydrogen. Hydrochloric acid, sometimes called muriatic acid, is a commonly used acid in industry and laboratories. The concentration of the chloride ion is 0.54 m; They also undergo redox reactions with metal halides in solution, displacing. Halogens react to a small extent with water, forming acidic solutions. Acidic Property Of Halogens In Water.
From chemisfast.blogspot.com
Halogen family elementspropertiesperiodic tableoxyacidsradioactivity. PG.CHEMEASY Acidic Property Of Halogens In Water Halogens react to a small extent with water, forming acidic solutions with bleaching properties. The concentration of the chloride ion is 0.54 m; It is a regular tetrahedral molecule, and reacts violently with water forming silicic acid and hydrochloric acid. The electronegativity of an element is a measurement of the strength of its atom in a molecule to pull electrons. Acidic Property Of Halogens In Water.
From slideplayer.com
Edexcel Additional Science C 2 ppt download Acidic Property Of Halogens In Water The electronegativity of an element is a measurement of the strength of its atom in a molecule to pull electrons towards its nucleus. It is useful as a raw material for the production of pure silicon,. Hydrohalic acids are commonly termed hydrogen halides when they dissolve in water to give acids. Halogens react to a small extent with water, forming. Acidic Property Of Halogens In Water.
From www.tes.com
Halogen Displacement Reactions GCSE AQA Teaching Resources Acidic Property Of Halogens In Water It is useful as a raw material for the production of pure silicon,. Hydrochloric acid, sometimes called muriatic acid, is a commonly used acid in industry and laboratories. Hydrogen fluoride, hydrogen chloride, hydrogen bromide and hydrogen. The concentration of the chloride ion is 0.54 m; The solubility decreases down the group. All of the halogens occur in seawater as halide. Acidic Property Of Halogens In Water.
From slideplayer.com
Reactions in Aqueous Solutions ppt download Acidic Property Of Halogens In Water They also undergo redox reactions with metal halides in solution, displacing. Halogens react to a small extent with water, forming acidic solutions with bleaching properties. Hydrochloric acid, sometimes called muriatic acid, is a commonly used acid in industry and laboratories. The concentration of the chloride ion is 0.54 m; The solubility decreases down the group. Chlorine, bromine and iodine are. Acidic Property Of Halogens In Water.
From www.slideserve.com
PPT Characteristic Properties of the Halogens PowerPoint Presentation ID370141 Acidic Property Of Halogens In Water They also undergo redox reactions with metal halides in solution, displacing. Chlorine, bromine and iodine are soluble in water to form an acidic solution. The solubility decreases down the group. This page discusses the acidity of the hydrogen halides: The electronegativity of an element is a measurement of the strength of its atom in a molecule to pull electrons towards. Acidic Property Of Halogens In Water.
From www.tes.com
Chemistry 7 Reactions and Uses of the Halogens (Slides and Student Led Tasks Acidic Property Of Halogens In Water Hydrochloric acid, sometimes called muriatic acid, is a commonly used acid in industry and laboratories. They also undergo redox reactions with metal halides in solution, displacing. The electronegativity of an element is a measurement of the strength of its atom in a molecule to pull electrons towards its nucleus. The solubility decreases down the group. Hydrogen fluoride, hydrogen chloride, hydrogen. Acidic Property Of Halogens In Water.
From ppt-online.org
Elements 17 (7A) group. Study of the properties of halogens and the determination of halide ions Acidic Property Of Halogens In Water Hydrochloric acid, sometimes called muriatic acid, is a commonly used acid in industry and laboratories. The electronegativity of an element is a measurement of the strength of its atom in a molecule to pull electrons towards its nucleus. This page discusses the acidity of the hydrogen halides: Hydrogen fluoride, hydrogen chloride, hydrogen bromide and hydrogen. Halogens react to a small. Acidic Property Of Halogens In Water.
From www.slideserve.com
PPT Group 7 the halogens PowerPoint Presentation, free download ID3005113 Acidic Property Of Halogens In Water Chlorine, bromine and iodine are soluble in water to form an acidic solution. Hydrochloric acid, sometimes called muriatic acid, is a commonly used acid in industry and laboratories. Halogens react to a small extent with water, forming acidic solutions with bleaching properties. The concentration of the chloride ion is 0.54 m; This page discusses the acidity of the hydrogen halides:. Acidic Property Of Halogens In Water.
From scienceinfo.com
Chemical Properties of Halogen Elements and Hydrogen Halides Acidic Property Of Halogens In Water The electronegativity of an element is a measurement of the strength of its atom in a molecule to pull electrons towards its nucleus. All of the halogens occur in seawater as halide ions. They also undergo redox reactions with metal halides in solution, displacing. This page discusses the acidity of the hydrogen halides: It is useful as a raw material. Acidic Property Of Halogens In Water.
From www.sliderbase.com
Group 7 Halogens Presentation Chemistry Acidic Property Of Halogens In Water The solubility decreases down the group. This page discusses the acidity of the hydrogen halides: Hydrogen fluoride, hydrogen chloride, hydrogen bromide and hydrogen. Halogens react to a small extent with water, forming acidic solutions with bleaching properties. They also undergo redox reactions with metal halides in solution, displacing. The concentration of the chloride ion is 0.54 m; All of the. Acidic Property Of Halogens In Water.
From www.slideserve.com
PPT Reactions of the halogens and halide ions PowerPoint Presentation ID2518013 Acidic Property Of Halogens In Water It is a regular tetrahedral molecule, and reacts violently with water forming silicic acid and hydrochloric acid. They also undergo redox reactions with metal halides in solution, displacing. The solubility decreases down the group. Hydrochloric acid, sometimes called muriatic acid, is a commonly used acid in industry and laboratories. This page discusses the acidity of the hydrogen halides: All of. Acidic Property Of Halogens In Water.
From www.slideserve.com
PPT Group 7 The Halogens PowerPoint Presentation, free download ID1275374 Acidic Property Of Halogens In Water Hydrohalic acids are commonly termed hydrogen halides when they dissolve in water to give acids. It is useful as a raw material for the production of pure silicon,. Hydrochloric acid, sometimes called muriatic acid, is a commonly used acid in industry and laboratories. The electronegativity of an element is a measurement of the strength of its atom in a molecule. Acidic Property Of Halogens In Water.
From www.slideserve.com
PPT Characteristic Properties of the Halogens PowerPoint Presentation ID370141 Acidic Property Of Halogens In Water Chlorine, bromine and iodine are soluble in water to form an acidic solution. The concentration of the chloride ion is 0.54 m; They also undergo redox reactions with metal halides in solution, displacing. It is a regular tetrahedral molecule, and reacts violently with water forming silicic acid and hydrochloric acid. The solubility decreases down the group. Hydrochloric acid, sometimes called. Acidic Property Of Halogens In Water.
From ppt-online.org
Study of the properties of halogens and the determination of halide ions in aqueous solution Acidic Property Of Halogens In Water They also undergo redox reactions with metal halides in solution, displacing. Hydrochloric acid, sometimes called muriatic acid, is a commonly used acid in industry and laboratories. Hydrohalic acids are commonly termed hydrogen halides when they dissolve in water to give acids. Halogens react to a small extent with water, forming acidic solutions with bleaching properties. The electronegativity of an element. Acidic Property Of Halogens In Water.