Error Analysis Titration Lab . Errors that cause the measured mean value (x bar) for any series of measurements to be displaced, in one. Errors in titration can result from inaccurate measurements, equipment imperfections, or human mistakes. First, there is an intrinsic error of the. For three or more point repetitions, do the average and standard deviation. There are several types of errors that can make titration result differ from the reality. Your laboratory report needs to include the following: The following discussion, the errors in a titration experiment are considered. The first section is a detailed look at how to determine the most. It entails consideration of potential. Titration error analysis is a vital aspect of quantitative chemistry, specifically in volumetric analysis.
from mmerevise.co.uk
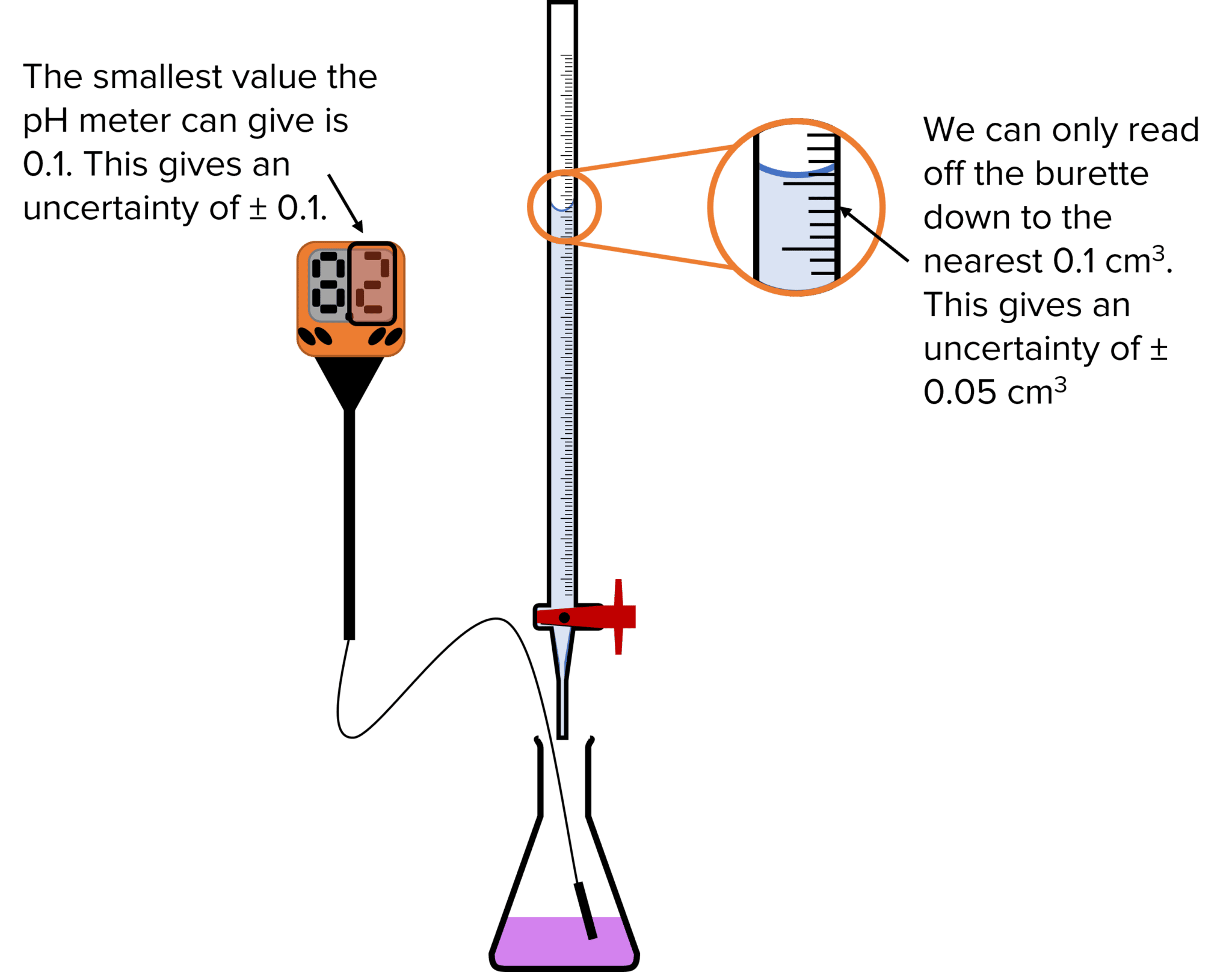
The following discussion, the errors in a titration experiment are considered. Titration error analysis is a vital aspect of quantitative chemistry, specifically in volumetric analysis. There are several types of errors that can make titration result differ from the reality. It entails consideration of potential. The first section is a detailed look at how to determine the most. Errors that cause the measured mean value (x bar) for any series of measurements to be displaced, in one. First, there is an intrinsic error of the. Errors in titration can result from inaccurate measurements, equipment imperfections, or human mistakes. Your laboratory report needs to include the following: For three or more point repetitions, do the average and standard deviation.
Titrations and Uncertainties MME
Error Analysis Titration Lab First, there is an intrinsic error of the. It entails consideration of potential. Errors in titration can result from inaccurate measurements, equipment imperfections, or human mistakes. Your laboratory report needs to include the following: Titration error analysis is a vital aspect of quantitative chemistry, specifically in volumetric analysis. The following discussion, the errors in a titration experiment are considered. For three or more point repetitions, do the average and standard deviation. First, there is an intrinsic error of the. There are several types of errors that can make titration result differ from the reality. The first section is a detailed look at how to determine the most. Errors that cause the measured mean value (x bar) for any series of measurements to be displaced, in one.
From theedge.com.hk
Chemistry How To Titration The Edge Error Analysis Titration Lab Errors that cause the measured mean value (x bar) for any series of measurements to be displaced, in one. First, there is an intrinsic error of the. Errors in titration can result from inaccurate measurements, equipment imperfections, or human mistakes. Your laboratory report needs to include the following: The following discussion, the errors in a titration experiment are considered. For. Error Analysis Titration Lab.
From itnewstoday.net
Fixed How To Fix Experimental Error Sources In Titration. IT News Today Error Analysis Titration Lab Errors in titration can result from inaccurate measurements, equipment imperfections, or human mistakes. Your laboratory report needs to include the following: There are several types of errors that can make titration result differ from the reality. It entails consideration of potential. Titration error analysis is a vital aspect of quantitative chemistry, specifically in volumetric analysis. The following discussion, the errors. Error Analysis Titration Lab.
From childhealthpolicy.vumc.org
🐈 Titration experiment results. How do you report a titration experiment?. 20221029 Error Analysis Titration Lab Errors that cause the measured mean value (x bar) for any series of measurements to be displaced, in one. Errors in titration can result from inaccurate measurements, equipment imperfections, or human mistakes. Titration error analysis is a vital aspect of quantitative chemistry, specifically in volumetric analysis. There are several types of errors that can make titration result differ from the. Error Analysis Titration Lab.
From childhealthpolicy.vumc.org
🐈 Titration experiment results. How do you report a titration experiment?. 20221029 Error Analysis Titration Lab There are several types of errors that can make titration result differ from the reality. First, there is an intrinsic error of the. Errors that cause the measured mean value (x bar) for any series of measurements to be displaced, in one. For three or more point repetitions, do the average and standard deviation. The first section is a detailed. Error Analysis Titration Lab.
From www.studocu.com
Error Analysis Example in Titration Chem 3300 Studocu Error Analysis Titration Lab Titration error analysis is a vital aspect of quantitative chemistry, specifically in volumetric analysis. It entails consideration of potential. First, there is an intrinsic error of the. Errors that cause the measured mean value (x bar) for any series of measurements to be displaced, in one. The first section is a detailed look at how to determine the most. The. Error Analysis Titration Lab.
From chemistrylabs-2.blogspot.com
Titration Apparatus Diagram Chemistry Labs Error Analysis Titration Lab Errors in titration can result from inaccurate measurements, equipment imperfections, or human mistakes. There are several types of errors that can make titration result differ from the reality. Your laboratory report needs to include the following: The following discussion, the errors in a titration experiment are considered. Titration error analysis is a vital aspect of quantitative chemistry, specifically in volumetric. Error Analysis Titration Lab.
From www.chegg.com
Solved Titration for Acetic Acid in VinegarLab Report Error Analysis Titration Lab The first section is a detailed look at how to determine the most. For three or more point repetitions, do the average and standard deviation. The following discussion, the errors in a titration experiment are considered. It entails consideration of potential. Your laboratory report needs to include the following: Errors that cause the measured mean value (x bar) for any. Error Analysis Titration Lab.
From www.vrogue.co
Northeastern University Chem 1215 Volumetric Analysis vrogue.co Error Analysis Titration Lab It entails consideration of potential. Errors in titration can result from inaccurate measurements, equipment imperfections, or human mistakes. First, there is an intrinsic error of the. The following discussion, the errors in a titration experiment are considered. Your laboratory report needs to include the following: For three or more point repetitions, do the average and standard deviation. The first section. Error Analysis Titration Lab.
From mmerevise.co.uk
Titrations and Uncertainties MME Error Analysis Titration Lab The first section is a detailed look at how to determine the most. Titration error analysis is a vital aspect of quantitative chemistry, specifically in volumetric analysis. Errors that cause the measured mean value (x bar) for any series of measurements to be displaced, in one. Errors in titration can result from inaccurate measurements, equipment imperfections, or human mistakes. The. Error Analysis Titration Lab.
From www.youtube.com
Titrations Analysis Percent Error Calculation YouTube Error Analysis Titration Lab The first section is a detailed look at how to determine the most. First, there is an intrinsic error of the. Titration error analysis is a vital aspect of quantitative chemistry, specifically in volumetric analysis. The following discussion, the errors in a titration experiment are considered. Errors that cause the measured mean value (x bar) for any series of measurements. Error Analysis Titration Lab.
From study.com
How to Accurately Perform Basic Error Analysis Lesson Error Analysis Titration Lab There are several types of errors that can make titration result differ from the reality. Titration error analysis is a vital aspect of quantitative chemistry, specifically in volumetric analysis. It entails consideration of potential. The first section is a detailed look at how to determine the most. Errors in titration can result from inaccurate measurements, equipment imperfections, or human mistakes.. Error Analysis Titration Lab.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Error Analysis Titration Lab Errors that cause the measured mean value (x bar) for any series of measurements to be displaced, in one. The following discussion, the errors in a titration experiment are considered. It entails consideration of potential. First, there is an intrinsic error of the. For three or more point repetitions, do the average and standard deviation. The first section is a. Error Analysis Titration Lab.
From studylib.net
AcidBase Titration Answers, Sources of Error Error Analysis Titration Lab For three or more point repetitions, do the average and standard deviation. Errors that cause the measured mean value (x bar) for any series of measurements to be displaced, in one. There are several types of errors that can make titration result differ from the reality. Your laboratory report needs to include the following: First, there is an intrinsic error. Error Analysis Titration Lab.
From www.youtube.com
Errors in titrations YouTube Error Analysis Titration Lab The first section is a detailed look at how to determine the most. First, there is an intrinsic error of the. The following discussion, the errors in a titration experiment are considered. Your laboratory report needs to include the following: For three or more point repetitions, do the average and standard deviation. There are several types of errors that can. Error Analysis Titration Lab.
From www.scribd.com
titration ( chemistry Experiment report) Titration Analysis Error Analysis Titration Lab Errors that cause the measured mean value (x bar) for any series of measurements to be displaced, in one. There are several types of errors that can make titration result differ from the reality. The first section is a detailed look at how to determine the most. It entails consideration of potential. The following discussion, the errors in a titration. Error Analysis Titration Lab.
From www.docsity.com
Experiment 1 Measuring and Error Analysis Sample Lab Report PHYS 217 Lab Reports Physics Error Analysis Titration Lab It entails consideration of potential. First, there is an intrinsic error of the. Errors that cause the measured mean value (x bar) for any series of measurements to be displaced, in one. Your laboratory report needs to include the following: The first section is a detailed look at how to determine the most. The following discussion, the errors in a. Error Analysis Titration Lab.
From www.scribd.com
Lab 1 (Error Analysis) PDF Volume Uncertainty Error Analysis Titration Lab First, there is an intrinsic error of the. Your laboratory report needs to include the following: The first section is a detailed look at how to determine the most. For three or more point repetitions, do the average and standard deviation. It entails consideration of potential. Errors that cause the measured mean value (x bar) for any series of measurements. Error Analysis Titration Lab.
From chemistnotes.com
Errors in Chemical Analysis Determinate and Indeterminate Errors Chemistry Notes Error Analysis Titration Lab Errors in titration can result from inaccurate measurements, equipment imperfections, or human mistakes. First, there is an intrinsic error of the. The first section is a detailed look at how to determine the most. It entails consideration of potential. For three or more point repetitions, do the average and standard deviation. Errors that cause the measured mean value (x bar). Error Analysis Titration Lab.
From mmerevise.co.uk
Titrations and Uncertainties MME Error Analysis Titration Lab Your laboratory report needs to include the following: The following discussion, the errors in a titration experiment are considered. Errors that cause the measured mean value (x bar) for any series of measurements to be displaced, in one. For three or more point repetitions, do the average and standard deviation. Titration error analysis is a vital aspect of quantitative chemistry,. Error Analysis Titration Lab.
From www.slideserve.com
PPT Volumetric Analysis (Titration) PowerPoint Presentation, free download ID2067899 Error Analysis Titration Lab Your laboratory report needs to include the following: It entails consideration of potential. Errors in titration can result from inaccurate measurements, equipment imperfections, or human mistakes. For three or more point repetitions, do the average and standard deviation. The first section is a detailed look at how to determine the most. The following discussion, the errors in a titration experiment. Error Analysis Titration Lab.
From www.youtube.com
Lab Report Error Analysis & Limits of the Model YouTube Error Analysis Titration Lab It entails consideration of potential. There are several types of errors that can make titration result differ from the reality. The first section is a detailed look at how to determine the most. Errors that cause the measured mean value (x bar) for any series of measurements to be displaced, in one. Your laboratory report needs to include the following:. Error Analysis Titration Lab.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Error Analysis Titration Lab There are several types of errors that can make titration result differ from the reality. Titration error analysis is a vital aspect of quantitative chemistry, specifically in volumetric analysis. The first section is a detailed look at how to determine the most. Errors that cause the measured mean value (x bar) for any series of measurements to be displaced, in. Error Analysis Titration Lab.
From www.scribd.com
Lab 2 Error Analysis PDF Uncertainty Standard Deviation Error Analysis Titration Lab Errors that cause the measured mean value (x bar) for any series of measurements to be displaced, in one. Your laboratory report needs to include the following: Titration error analysis is a vital aspect of quantitative chemistry, specifically in volumetric analysis. Errors in titration can result from inaccurate measurements, equipment imperfections, or human mistakes. There are several types of errors. Error Analysis Titration Lab.
From beyondlabz.freshdesk.com
Titrations Lab Overview Beyond Labz Error Analysis Titration Lab The first section is a detailed look at how to determine the most. Titration error analysis is a vital aspect of quantitative chemistry, specifically in volumetric analysis. Your laboratory report needs to include the following: For three or more point repetitions, do the average and standard deviation. There are several types of errors that can make titration result differ from. Error Analysis Titration Lab.
From www.vecteezy.com
Acid base titration experiment and phases of color change during titration 20240684 Vector Art Error Analysis Titration Lab There are several types of errors that can make titration result differ from the reality. Titration error analysis is a vital aspect of quantitative chemistry, specifically in volumetric analysis. The first section is a detailed look at how to determine the most. First, there is an intrinsic error of the. Errors in titration can result from inaccurate measurements, equipment imperfections,. Error Analysis Titration Lab.
From courses.lumenlearning.com
Quantitative Chemical Analysis Chemistry Error Analysis Titration Lab There are several types of errors that can make titration result differ from the reality. Errors that cause the measured mean value (x bar) for any series of measurements to be displaced, in one. It entails consideration of potential. For three or more point repetitions, do the average and standard deviation. Errors in titration can result from inaccurate measurements, equipment. Error Analysis Titration Lab.
From mungfali.com
Acid Base Titration Experiment Error Analysis Titration Lab For three or more point repetitions, do the average and standard deviation. First, there is an intrinsic error of the. Errors in titration can result from inaccurate measurements, equipment imperfections, or human mistakes. The following discussion, the errors in a titration experiment are considered. The first section is a detailed look at how to determine the most. There are several. Error Analysis Titration Lab.
From www.answersarena.com
[Solved] Review the list of common titration errors. Dete Error Analysis Titration Lab First, there is an intrinsic error of the. It entails consideration of potential. Titration error analysis is a vital aspect of quantitative chemistry, specifically in volumetric analysis. The following discussion, the errors in a titration experiment are considered. There are several types of errors that can make titration result differ from the reality. The first section is a detailed look. Error Analysis Titration Lab.
From www.slideserve.com
PPT Titrations PowerPoint Presentation ID2145156 Error Analysis Titration Lab The first section is a detailed look at how to determine the most. Errors in titration can result from inaccurate measurements, equipment imperfections, or human mistakes. Titration error analysis is a vital aspect of quantitative chemistry, specifically in volumetric analysis. The following discussion, the errors in a titration experiment are considered. First, there is an intrinsic error of the. Your. Error Analysis Titration Lab.
From www.scribd.com
LAB Report 3 Titration Chemistry Error Analysis Titration Lab It entails consideration of potential. Errors in titration can result from inaccurate measurements, equipment imperfections, or human mistakes. There are several types of errors that can make titration result differ from the reality. The following discussion, the errors in a titration experiment are considered. Errors that cause the measured mean value (x bar) for any series of measurements to be. Error Analysis Titration Lab.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Error Analysis Titration Lab Errors in titration can result from inaccurate measurements, equipment imperfections, or human mistakes. Your laboratory report needs to include the following: First, there is an intrinsic error of the. The first section is a detailed look at how to determine the most. Titration error analysis is a vital aspect of quantitative chemistry, specifically in volumetric analysis. For three or more. Error Analysis Titration Lab.
From www.expii.com
Types of Error — Overview & Comparison Expii Error Analysis Titration Lab There are several types of errors that can make titration result differ from the reality. The following discussion, the errors in a titration experiment are considered. It entails consideration of potential. The first section is a detailed look at how to determine the most. Errors that cause the measured mean value (x bar) for any series of measurements to be. Error Analysis Titration Lab.
From about.dataclassroom.com
AcidBase Titration Lab — DataClassroom Error Analysis Titration Lab Errors that cause the measured mean value (x bar) for any series of measurements to be displaced, in one. Titration error analysis is a vital aspect of quantitative chemistry, specifically in volumetric analysis. It entails consideration of potential. Your laboratory report needs to include the following: The first section is a detailed look at how to determine the most. There. Error Analysis Titration Lab.
From www.microlit.com
An Advanced Guide to Titration Microlit Error Analysis Titration Lab Errors that cause the measured mean value (x bar) for any series of measurements to be displaced, in one. There are several types of errors that can make titration result differ from the reality. First, there is an intrinsic error of the. Titration error analysis is a vital aspect of quantitative chemistry, specifically in volumetric analysis. The first section is. Error Analysis Titration Lab.
From www.youtube.com
How to calculate uncertainty in titration YouTube Error Analysis Titration Lab Your laboratory report needs to include the following: Errors in titration can result from inaccurate measurements, equipment imperfections, or human mistakes. For three or more point repetitions, do the average and standard deviation. Titration error analysis is a vital aspect of quantitative chemistry, specifically in volumetric analysis. Errors that cause the measured mean value (x bar) for any series of. Error Analysis Titration Lab.