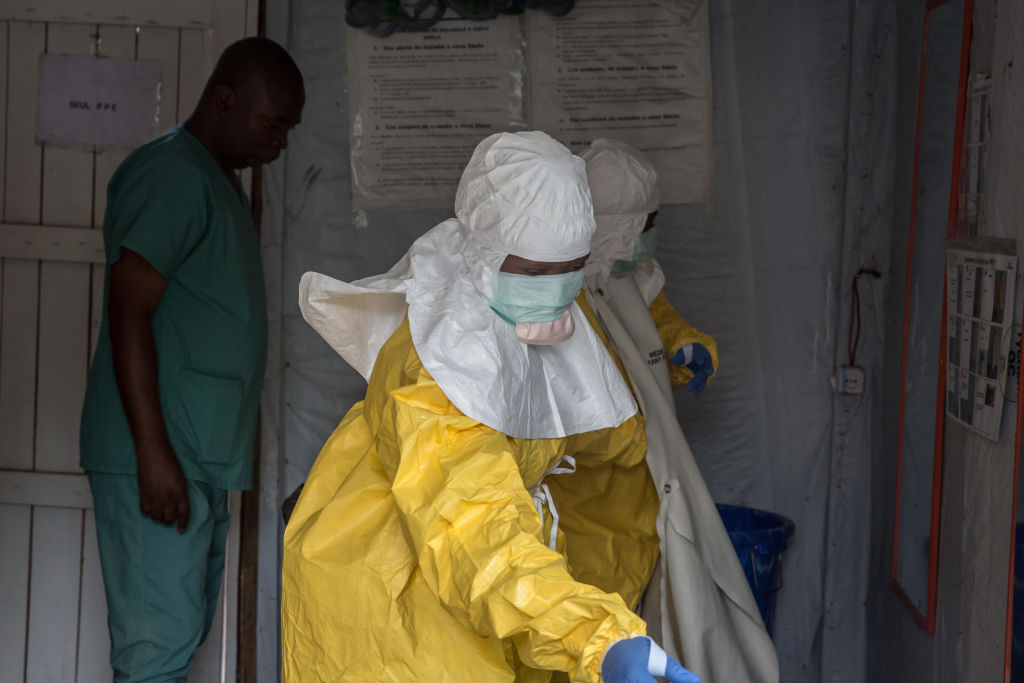
Cdc Ebola Lab Testing . In the united states, presumptive testing for ebola virus (zaire ebolavirus) is available at 69 laboratory response network (lrn). Guidance to assist state and local health departments and acute care hospitals as they develop preparedness plans for patients. Ideally, specimens should be taken when a symptomatic patient reports to a healthcare facility and is suspected of having an ebola. Clinical teams should coordinate with public health officials and cdc to assess the risk of ebola disease based on the clinical. On wednesday, december 7, 2022, the u.s. Interim guidance for specimen collection, transport, testing, and submission for patients with suspected infection with ebola. Learn how clinical and public health laboratories collaborate with healthcare teams to provide the testing required to diagnose and treat hospital. Centers for disease control and prevention (cdc) updated the guidance for the collection,. Several diagnostic tests are available for detection of ebola virus disease.
from time.com
Clinical teams should coordinate with public health officials and cdc to assess the risk of ebola disease based on the clinical. In the united states, presumptive testing for ebola virus (zaire ebolavirus) is available at 69 laboratory response network (lrn). On wednesday, december 7, 2022, the u.s. Several diagnostic tests are available for detection of ebola virus disease. Guidance to assist state and local health departments and acute care hospitals as they develop preparedness plans for patients. Ideally, specimens should be taken when a symptomatic patient reports to a healthcare facility and is suspected of having an ebola. Interim guidance for specimen collection, transport, testing, and submission for patients with suspected infection with ebola. Learn how clinical and public health laboratories collaborate with healthcare teams to provide the testing required to diagnose and treat hospital. Centers for disease control and prevention (cdc) updated the guidance for the collection,.
Two Ebola Treatments Show Promise, CDC Says TIME
Cdc Ebola Lab Testing Ideally, specimens should be taken when a symptomatic patient reports to a healthcare facility and is suspected of having an ebola. Several diagnostic tests are available for detection of ebola virus disease. In the united states, presumptive testing for ebola virus (zaire ebolavirus) is available at 69 laboratory response network (lrn). On wednesday, december 7, 2022, the u.s. Guidance to assist state and local health departments and acute care hospitals as they develop preparedness plans for patients. Interim guidance for specimen collection, transport, testing, and submission for patients with suspected infection with ebola. Centers for disease control and prevention (cdc) updated the guidance for the collection,. Learn how clinical and public health laboratories collaborate with healthcare teams to provide the testing required to diagnose and treat hospital. Clinical teams should coordinate with public health officials and cdc to assess the risk of ebola disease based on the clinical. Ideally, specimens should be taken when a symptomatic patient reports to a healthcare facility and is suspected of having an ebola.
From www.axios.com
tests confirm new Ebola outbreak in the Congo Cdc Ebola Lab Testing Interim guidance for specimen collection, transport, testing, and submission for patients with suspected infection with ebola. Centers for disease control and prevention (cdc) updated the guidance for the collection,. Several diagnostic tests are available for detection of ebola virus disease. In the united states, presumptive testing for ebola virus (zaire ebolavirus) is available at 69 laboratory response network (lrn). On. Cdc Ebola Lab Testing.
From www.washingtonpost.com
The lessons of the Ebola outbreak suggest a larger, faster response is Cdc Ebola Lab Testing Clinical teams should coordinate with public health officials and cdc to assess the risk of ebola disease based on the clinical. Ideally, specimens should be taken when a symptomatic patient reports to a healthcare facility and is suspected of having an ebola. In the united states, presumptive testing for ebola virus (zaire ebolavirus) is available at 69 laboratory response network. Cdc Ebola Lab Testing.
From www.bbc.com
How disease testing tech is saving lives faster BBC News Cdc Ebola Lab Testing Centers for disease control and prevention (cdc) updated the guidance for the collection,. Ideally, specimens should be taken when a symptomatic patient reports to a healthcare facility and is suspected of having an ebola. Interim guidance for specimen collection, transport, testing, and submission for patients with suspected infection with ebola. Guidance to assist state and local health departments and acute. Cdc Ebola Lab Testing.
From www.usatoday.com
CDC chief says Ebola must be contained in Africa Cdc Ebola Lab Testing Interim guidance for specimen collection, transport, testing, and submission for patients with suspected infection with ebola. Ideally, specimens should be taken when a symptomatic patient reports to a healthcare facility and is suspected of having an ebola. In the united states, presumptive testing for ebola virus (zaire ebolavirus) is available at 69 laboratory response network (lrn). Centers for disease control. Cdc Ebola Lab Testing.
From www.military.com
Army Biowarfare Lab that Tests Ebola and the Plague Failed Safety Cdc Ebola Lab Testing Centers for disease control and prevention (cdc) updated the guidance for the collection,. Guidance to assist state and local health departments and acute care hospitals as they develop preparedness plans for patients. Several diagnostic tests are available for detection of ebola virus disease. In the united states, presumptive testing for ebola virus (zaire ebolavirus) is available at 69 laboratory response. Cdc Ebola Lab Testing.
From qz.com
This is what it’s like inside an Ebola testing lab in Mali — Quartz Cdc Ebola Lab Testing Learn how clinical and public health laboratories collaborate with healthcare teams to provide the testing required to diagnose and treat hospital. Ideally, specimens should be taken when a symptomatic patient reports to a healthcare facility and is suspected of having an ebola. Clinical teams should coordinate with public health officials and cdc to assess the risk of ebola disease based. Cdc Ebola Lab Testing.
From ecdc.europa.eu
Algorithm for laboratory diagnosis of Ebola virus disease Cdc Ebola Lab Testing Clinical teams should coordinate with public health officials and cdc to assess the risk of ebola disease based on the clinical. On wednesday, december 7, 2022, the u.s. Centers for disease control and prevention (cdc) updated the guidance for the collection,. Guidance to assist state and local health departments and acute care hospitals as they develop preparedness plans for patients.. Cdc Ebola Lab Testing.
From usatoday.com
Lab error at CDC may have caused Ebola exposure Cdc Ebola Lab Testing Learn how clinical and public health laboratories collaborate with healthcare teams to provide the testing required to diagnose and treat hospital. In the united states, presumptive testing for ebola virus (zaire ebolavirus) is available at 69 laboratory response network (lrn). Centers for disease control and prevention (cdc) updated the guidance for the collection,. On wednesday, december 7, 2022, the u.s.. Cdc Ebola Lab Testing.
From www.bbc.com
US lab worker monitored for Ebola BBC News Cdc Ebola Lab Testing Several diagnostic tests are available for detection of ebola virus disease. Guidance to assist state and local health departments and acute care hospitals as they develop preparedness plans for patients. Clinical teams should coordinate with public health officials and cdc to assess the risk of ebola disease based on the clinical. Ideally, specimens should be taken when a symptomatic patient. Cdc Ebola Lab Testing.
From peninsulapress.com
Stanford professor testing cancer drug for possible Ebola treatment Cdc Ebola Lab Testing Learn how clinical and public health laboratories collaborate with healthcare teams to provide the testing required to diagnose and treat hospital. Guidance to assist state and local health departments and acute care hospitals as they develop preparedness plans for patients. Interim guidance for specimen collection, transport, testing, and submission for patients with suspected infection with ebola. Several diagnostic tests are. Cdc Ebola Lab Testing.
From abc7chicago.com
The CDC answers your questions about Ebola ABC7 Chicago Cdc Ebola Lab Testing On wednesday, december 7, 2022, the u.s. Learn how clinical and public health laboratories collaborate with healthcare teams to provide the testing required to diagnose and treat hospital. Clinical teams should coordinate with public health officials and cdc to assess the risk of ebola disease based on the clinical. Centers for disease control and prevention (cdc) updated the guidance for. Cdc Ebola Lab Testing.
From www.cbsnews.com
CDC releases new Ebola guidelines CBS News Cdc Ebola Lab Testing Clinical teams should coordinate with public health officials and cdc to assess the risk of ebola disease based on the clinical. Interim guidance for specimen collection, transport, testing, and submission for patients with suspected infection with ebola. Guidance to assist state and local health departments and acute care hospitals as they develop preparedness plans for patients. Ideally, specimens should be. Cdc Ebola Lab Testing.
From www.cleveland.com
CDC monitoring tech for possible Ebola exposure Cdc Ebola Lab Testing Centers for disease control and prevention (cdc) updated the guidance for the collection,. Interim guidance for specimen collection, transport, testing, and submission for patients with suspected infection with ebola. In the united states, presumptive testing for ebola virus (zaire ebolavirus) is available at 69 laboratory response network (lrn). Guidance to assist state and local health departments and acute care hospitals. Cdc Ebola Lab Testing.
From www.usatoday.com
CDC says people at high risk for Ebola should stay home Cdc Ebola Lab Testing Clinical teams should coordinate with public health officials and cdc to assess the risk of ebola disease based on the clinical. On wednesday, december 7, 2022, the u.s. In the united states, presumptive testing for ebola virus (zaire ebolavirus) is available at 69 laboratory response network (lrn). Guidance to assist state and local health departments and acute care hospitals as. Cdc Ebola Lab Testing.
From www.washingtonpost.com
CDC seeks new labs for bioterror pathogens to replace aging facility Cdc Ebola Lab Testing Interim guidance for specimen collection, transport, testing, and submission for patients with suspected infection with ebola. Centers for disease control and prevention (cdc) updated the guidance for the collection,. On wednesday, december 7, 2022, the u.s. In the united states, presumptive testing for ebola virus (zaire ebolavirus) is available at 69 laboratory response network (lrn). Clinical teams should coordinate with. Cdc Ebola Lab Testing.
From abc7news.com
CDC says lab tech in Atlanta may have accidentally been exposed to Cdc Ebola Lab Testing In the united states, presumptive testing for ebola virus (zaire ebolavirus) is available at 69 laboratory response network (lrn). Interim guidance for specimen collection, transport, testing, and submission for patients with suspected infection with ebola. On wednesday, december 7, 2022, the u.s. Several diagnostic tests are available for detection of ebola virus disease. Ideally, specimens should be taken when a. Cdc Ebola Lab Testing.
From www.medpagetoday.com
Ebola Lab Net Ready to Test Medpage Today Cdc Ebola Lab Testing Learn how clinical and public health laboratories collaborate with healthcare teams to provide the testing required to diagnose and treat hospital. Clinical teams should coordinate with public health officials and cdc to assess the risk of ebola disease based on the clinical. On wednesday, december 7, 2022, the u.s. Several diagnostic tests are available for detection of ebola virus disease.. Cdc Ebola Lab Testing.
From www.wsj.com
CDC Battling Ebola by Training Medical Volunteers Cdc Ebola Lab Testing Guidance to assist state and local health departments and acute care hospitals as they develop preparedness plans for patients. Clinical teams should coordinate with public health officials and cdc to assess the risk of ebola disease based on the clinical. Several diagnostic tests are available for detection of ebola virus disease. Interim guidance for specimen collection, transport, testing, and submission. Cdc Ebola Lab Testing.
From www.washingtonpost.com
CDC reports potential Ebola exposure in Atlanta lab The Washington Post Cdc Ebola Lab Testing Learn how clinical and public health laboratories collaborate with healthcare teams to provide the testing required to diagnose and treat hospital. Guidance to assist state and local health departments and acute care hospitals as they develop preparedness plans for patients. On wednesday, december 7, 2022, the u.s. Ideally, specimens should be taken when a symptomatic patient reports to a healthcare. Cdc Ebola Lab Testing.
From edition.cnn.com
Ebola Five ways the CDC got it wrong CNN Cdc Ebola Lab Testing Interim guidance for specimen collection, transport, testing, and submission for patients with suspected infection with ebola. On wednesday, december 7, 2022, the u.s. In the united states, presumptive testing for ebola virus (zaire ebolavirus) is available at 69 laboratory response network (lrn). Learn how clinical and public health laboratories collaborate with healthcare teams to provide the testing required to diagnose. Cdc Ebola Lab Testing.
From www.express.co.uk
Image 5 The Ebola Crisis Pictures Pics Express.co.uk Cdc Ebola Lab Testing Several diagnostic tests are available for detection of ebola virus disease. Centers for disease control and prevention (cdc) updated the guidance for the collection,. Learn how clinical and public health laboratories collaborate with healthcare teams to provide the testing required to diagnose and treat hospital. In the united states, presumptive testing for ebola virus (zaire ebolavirus) is available at 69. Cdc Ebola Lab Testing.
From edition.cnn.com
Experimental Ebola treatments show promise in lab study CNN Cdc Ebola Lab Testing Clinical teams should coordinate with public health officials and cdc to assess the risk of ebola disease based on the clinical. Several diagnostic tests are available for detection of ebola virus disease. On wednesday, december 7, 2022, the u.s. Interim guidance for specimen collection, transport, testing, and submission for patients with suspected infection with ebola. Ideally, specimens should be taken. Cdc Ebola Lab Testing.
From www.today.com
Get a rare look inside US Army’s Ebola lab Cdc Ebola Lab Testing Ideally, specimens should be taken when a symptomatic patient reports to a healthcare facility and is suspected of having an ebola. Learn how clinical and public health laboratories collaborate with healthcare teams to provide the testing required to diagnose and treat hospital. On wednesday, december 7, 2022, the u.s. In the united states, presumptive testing for ebola virus (zaire ebolavirus). Cdc Ebola Lab Testing.
From www.foxnews.com
CDC releases revised Ebola guidelines for medical workers Fox News Video Cdc Ebola Lab Testing Learn how clinical and public health laboratories collaborate with healthcare teams to provide the testing required to diagnose and treat hospital. Ideally, specimens should be taken when a symptomatic patient reports to a healthcare facility and is suspected of having an ebola. Interim guidance for specimen collection, transport, testing, and submission for patients with suspected infection with ebola. Guidance to. Cdc Ebola Lab Testing.
From kuow.org
15Minute Ebola Test Approved For Fighting The Epidemic KUOW News and Cdc Ebola Lab Testing Guidance to assist state and local health departments and acute care hospitals as they develop preparedness plans for patients. On wednesday, december 7, 2022, the u.s. Clinical teams should coordinate with public health officials and cdc to assess the risk of ebola disease based on the clinical. Ideally, specimens should be taken when a symptomatic patient reports to a healthcare. Cdc Ebola Lab Testing.
From www.cnn.com
Texas deputy discharged after negative Ebola test CNN Cdc Ebola Lab Testing Centers for disease control and prevention (cdc) updated the guidance for the collection,. On wednesday, december 7, 2022, the u.s. In the united states, presumptive testing for ebola virus (zaire ebolavirus) is available at 69 laboratory response network (lrn). Ideally, specimens should be taken when a symptomatic patient reports to a healthcare facility and is suspected of having an ebola.. Cdc Ebola Lab Testing.
From www.washingtonpost.com
CDC issues formal guidelines giving workers more protection against Cdc Ebola Lab Testing Learn how clinical and public health laboratories collaborate with healthcare teams to provide the testing required to diagnose and treat hospital. Clinical teams should coordinate with public health officials and cdc to assess the risk of ebola disease based on the clinical. In the united states, presumptive testing for ebola virus (zaire ebolavirus) is available at 69 laboratory response network. Cdc Ebola Lab Testing.
From www.bbc.com
Ebola virus UK medics test readiness for outbreak BBC News Cdc Ebola Lab Testing Clinical teams should coordinate with public health officials and cdc to assess the risk of ebola disease based on the clinical. Guidance to assist state and local health departments and acute care hospitals as they develop preparedness plans for patients. Learn how clinical and public health laboratories collaborate with healthcare teams to provide the testing required to diagnose and treat. Cdc Ebola Lab Testing.
From time.com
Two Ebola Treatments Show Promise, CDC Says TIME Cdc Ebola Lab Testing Interim guidance for specimen collection, transport, testing, and submission for patients with suspected infection with ebola. Clinical teams should coordinate with public health officials and cdc to assess the risk of ebola disease based on the clinical. Several diagnostic tests are available for detection of ebola virus disease. Ideally, specimens should be taken when a symptomatic patient reports to a. Cdc Ebola Lab Testing.
From www.newsweek.com
FDA Approves Emergency Diagnostic Test for Ebola Patients Newsweek Cdc Ebola Lab Testing Learn how clinical and public health laboratories collaborate with healthcare teams to provide the testing required to diagnose and treat hospital. Clinical teams should coordinate with public health officials and cdc to assess the risk of ebola disease based on the clinical. Interim guidance for specimen collection, transport, testing, and submission for patients with suspected infection with ebola. In the. Cdc Ebola Lab Testing.
From www.washingtonpost.com
CDC reports potential Ebola exposure in Atlanta lab The Washington Post Cdc Ebola Lab Testing Learn how clinical and public health laboratories collaborate with healthcare teams to provide the testing required to diagnose and treat hospital. Centers for disease control and prevention (cdc) updated the guidance for the collection,. On wednesday, december 7, 2022, the u.s. Several diagnostic tests are available for detection of ebola virus disease. Guidance to assist state and local health departments. Cdc Ebola Lab Testing.
From www.usatoday.com
Lab error at CDC may have caused Ebola exposure Cdc Ebola Lab Testing On wednesday, december 7, 2022, the u.s. Guidance to assist state and local health departments and acute care hospitals as they develop preparedness plans for patients. Clinical teams should coordinate with public health officials and cdc to assess the risk of ebola disease based on the clinical. Learn how clinical and public health laboratories collaborate with healthcare teams to provide. Cdc Ebola Lab Testing.
From www.usatoday.com
Latest Ebola fear Safety of lab equipment Cdc Ebola Lab Testing Clinical teams should coordinate with public health officials and cdc to assess the risk of ebola disease based on the clinical. In the united states, presumptive testing for ebola virus (zaire ebolavirus) is available at 69 laboratory response network (lrn). Guidance to assist state and local health departments and acute care hospitals as they develop preparedness plans for patients. Centers. Cdc Ebola Lab Testing.
From www.cbsnews.com
More studies needed on contagious Ebola virus and bodily fluids CBS News Cdc Ebola Lab Testing Clinical teams should coordinate with public health officials and cdc to assess the risk of ebola disease based on the clinical. Ideally, specimens should be taken when a symptomatic patient reports to a healthcare facility and is suspected of having an ebola. On wednesday, december 7, 2022, the u.s. Several diagnostic tests are available for detection of ebola virus disease.. Cdc Ebola Lab Testing.
From www.healio.com
CDC lab tests show Ebola treatments being used in outbreak are effective Cdc Ebola Lab Testing On wednesday, december 7, 2022, the u.s. Several diagnostic tests are available for detection of ebola virus disease. Centers for disease control and prevention (cdc) updated the guidance for the collection,. Interim guidance for specimen collection, transport, testing, and submission for patients with suspected infection with ebola. In the united states, presumptive testing for ebola virus (zaire ebolavirus) is available. Cdc Ebola Lab Testing.