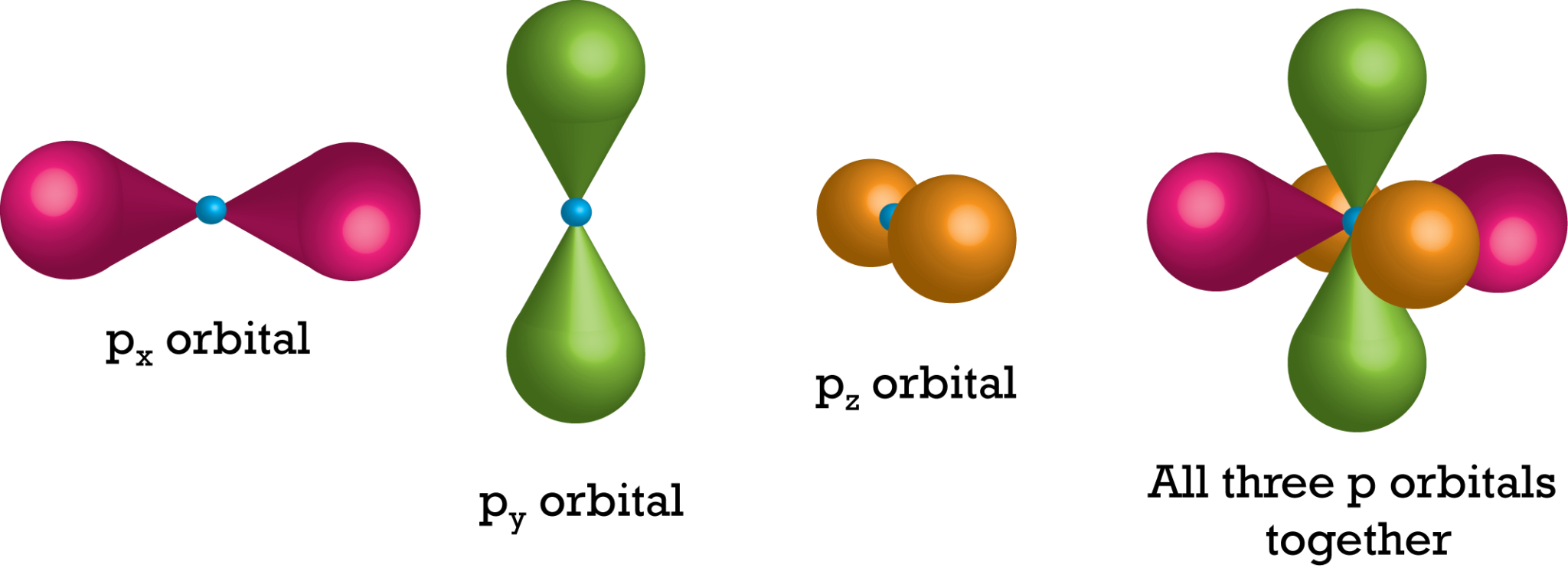
Is An Orbital The Same As A Shell . Shell refers to an energy level in an atom containing electrons, while an orbital is a region within a shell where electrons are likely to be found. Each atomic orbital can be occupied by a maximum of two electrons. Within a shell (same n n), all. Learn about orbitals, the three dimensional descriptions of electron locations around atoms, and how they combine to form bonds. Orbitals with the same principal quantum number and the same l value belong to the same subshell. Subshell is the pathway in which an electron. Orbitals exist at specific energy levels and electrons can only be found at these specific levels, not in between them. Shell is the pathway followed by electrons around an atom’s nucleus. Review the types of orbitals (s, p, d, f) and their. H, he +, li +2, etc.) the energy of each orbital within a particular shell is identical. All electrons that have the same value for n n (the principle quantum number) are in the same shell.
from chemistryskills.com
Review the types of orbitals (s, p, d, f) and their. Shell refers to an energy level in an atom containing electrons, while an orbital is a region within a shell where electrons are likely to be found. Within a shell (same n n), all. Each atomic orbital can be occupied by a maximum of two electrons. Subshell is the pathway in which an electron. Shell is the pathway followed by electrons around an atom’s nucleus. H, he +, li +2, etc.) the energy of each orbital within a particular shell is identical. Learn about orbitals, the three dimensional descriptions of electron locations around atoms, and how they combine to form bonds. Orbitals with the same principal quantum number and the same l value belong to the same subshell. Orbitals exist at specific energy levels and electrons can only be found at these specific levels, not in between them.
Shapes of Orbitals and their Types Chemistry Skills
Is An Orbital The Same As A Shell Learn about orbitals, the three dimensional descriptions of electron locations around atoms, and how they combine to form bonds. H, he +, li +2, etc.) the energy of each orbital within a particular shell is identical. Subshell is the pathway in which an electron. Each atomic orbital can be occupied by a maximum of two electrons. Review the types of orbitals (s, p, d, f) and their. Orbitals with the same principal quantum number and the same l value belong to the same subshell. Within a shell (same n n), all. Orbitals exist at specific energy levels and electrons can only be found at these specific levels, not in between them. Shell refers to an energy level in an atom containing electrons, while an orbital is a region within a shell where electrons are likely to be found. Learn about orbitals, the three dimensional descriptions of electron locations around atoms, and how they combine to form bonds. All electrons that have the same value for n n (the principle quantum number) are in the same shell. Shell is the pathway followed by electrons around an atom’s nucleus.
From www.vedantu.com
Distribution of Electrons in Different Orbits/Shells Learn Important Is An Orbital The Same As A Shell Shell refers to an energy level in an atom containing electrons, while an orbital is a region within a shell where electrons are likely to be found. All electrons that have the same value for n n (the principle quantum number) are in the same shell. Each atomic orbital can be occupied by a maximum of two electrons. Learn about. Is An Orbital The Same As A Shell.
From byjus.com
what is the sequence of shell , subshell, orbitals , orbits , electrons Is An Orbital The Same As A Shell Orbitals with the same principal quantum number and the same l value belong to the same subshell. Each atomic orbital can be occupied by a maximum of two electrons. All electrons that have the same value for n n (the principle quantum number) are in the same shell. H, he +, li +2, etc.) the energy of each orbital within. Is An Orbital The Same As A Shell.
From mavink.com
Electron Orbitals Explained Is An Orbital The Same As A Shell Orbitals with the same principal quantum number and the same l value belong to the same subshell. Within a shell (same n n), all. Orbitals exist at specific energy levels and electrons can only be found at these specific levels, not in between them. Each atomic orbital can be occupied by a maximum of two electrons. Subshell is the pathway. Is An Orbital The Same As A Shell.
From ybkmtmyrda.blogspot.com
How Many Orbitals Are In The N=3 Shell An s subshell only has one Is An Orbital The Same As A Shell Orbitals exist at specific energy levels and electrons can only be found at these specific levels, not in between them. Learn about orbitals, the three dimensional descriptions of electron locations around atoms, and how they combine to form bonds. Each atomic orbital can be occupied by a maximum of two electrons. Shell is the pathway followed by electrons around an. Is An Orbital The Same As A Shell.
From mavink.com
Electron Shells And Orbitals Is An Orbital The Same As A Shell Shell is the pathway followed by electrons around an atom’s nucleus. Orbitals exist at specific energy levels and electrons can only be found at these specific levels, not in between them. Subshell is the pathway in which an electron. Each atomic orbital can be occupied by a maximum of two electrons. Shell refers to an energy level in an atom. Is An Orbital The Same As A Shell.
From www.slideserve.com
PPT Chapter Three PowerPoint Presentation, free download ID5840496 Is An Orbital The Same As A Shell Orbitals with the same principal quantum number and the same l value belong to the same subshell. Shell refers to an energy level in an atom containing electrons, while an orbital is a region within a shell where electrons are likely to be found. Each atomic orbital can be occupied by a maximum of two electrons. Review the types of. Is An Orbital The Same As A Shell.
From courses.lumenlearning.com
Development of Quantum Theory Chemistry I Is An Orbital The Same As A Shell Within a shell (same n n), all. All electrons that have the same value for n n (the principle quantum number) are in the same shell. Each atomic orbital can be occupied by a maximum of two electrons. Orbitals exist at specific energy levels and electrons can only be found at these specific levels, not in between them. H, he. Is An Orbital The Same As A Shell.
From mungfali.com
Orbital Subshell Chart Is An Orbital The Same As A Shell Shell is the pathway followed by electrons around an atom’s nucleus. Learn about orbitals, the three dimensional descriptions of electron locations around atoms, and how they combine to form bonds. Orbitals exist at specific energy levels and electrons can only be found at these specific levels, not in between them. All electrons that have the same value for n n. Is An Orbital The Same As A Shell.
From newtondesk.com
Periodic Elements Electron Shells, SubShells, and Orbitals Chemistry Is An Orbital The Same As A Shell Each atomic orbital can be occupied by a maximum of two electrons. Review the types of orbitals (s, p, d, f) and their. Subshell is the pathway in which an electron. All electrons that have the same value for n n (the principle quantum number) are in the same shell. Learn about orbitals, the three dimensional descriptions of electron locations. Is An Orbital The Same As A Shell.
From www.youtube.com
Elements, Atoms, Shells, Subshells And Orbitals YouTube Is An Orbital The Same As A Shell Shell refers to an energy level in an atom containing electrons, while an orbital is a region within a shell where electrons are likely to be found. Review the types of orbitals (s, p, d, f) and their. Learn about orbitals, the three dimensional descriptions of electron locations around atoms, and how they combine to form bonds. Each atomic orbital. Is An Orbital The Same As A Shell.
From chem.libretexts.org
6.6 3D Representation of Orbitals Chemistry LibreTexts Is An Orbital The Same As A Shell All electrons that have the same value for n n (the principle quantum number) are in the same shell. Review the types of orbitals (s, p, d, f) and their. Shell is the pathway followed by electrons around an atom’s nucleus. Orbitals with the same principal quantum number and the same l value belong to the same subshell. Orbitals exist. Is An Orbital The Same As A Shell.
From www.teachoo.com
Distribution of Electrons in Different Orbits [with Examples] Teacho Is An Orbital The Same As A Shell Review the types of orbitals (s, p, d, f) and their. Each atomic orbital can be occupied by a maximum of two electrons. All electrons that have the same value for n n (the principle quantum number) are in the same shell. Shell refers to an energy level in an atom containing electrons, while an orbital is a region within. Is An Orbital The Same As A Shell.
From www.thoughtco.com
Electron Configuration Chart Is An Orbital The Same As A Shell Shell refers to an energy level in an atom containing electrons, while an orbital is a region within a shell where electrons are likely to be found. Learn about orbitals, the three dimensional descriptions of electron locations around atoms, and how they combine to form bonds. H, he +, li +2, etc.) the energy of each orbital within a particular. Is An Orbital The Same As A Shell.
From www.youtube.com
What is Difference between Shell, Sub shell and Orbital Structure Is An Orbital The Same As A Shell Review the types of orbitals (s, p, d, f) and their. Each atomic orbital can be occupied by a maximum of two electrons. Orbitals exist at specific energy levels and electrons can only be found at these specific levels, not in between them. Subshell is the pathway in which an electron. Within a shell (same n n), all. All electrons. Is An Orbital The Same As A Shell.
From www.chemistrystudent.com
Electron Orbitals (ALevel) ChemistryStudent Is An Orbital The Same As A Shell Learn about orbitals, the three dimensional descriptions of electron locations around atoms, and how they combine to form bonds. Shell is the pathway followed by electrons around an atom’s nucleus. Orbitals with the same principal quantum number and the same l value belong to the same subshell. Shell refers to an energy level in an atom containing electrons, while an. Is An Orbital The Same As A Shell.
From www.pinterest.com
Atoms, shells ,Subshells and Orbitals Atom, Submarine, Shells Is An Orbital The Same As A Shell Shell refers to an energy level in an atom containing electrons, while an orbital is a region within a shell where electrons are likely to be found. Learn about orbitals, the three dimensional descriptions of electron locations around atoms, and how they combine to form bonds. Within a shell (same n n), all. Orbitals exist at specific energy levels and. Is An Orbital The Same As A Shell.
From www.britannica.com
Chemical bonding Atomic Orbitals, Shapes, Hybridization Britannica Is An Orbital The Same As A Shell Review the types of orbitals (s, p, d, f) and their. Within a shell (same n n), all. Subshell is the pathway in which an electron. Orbitals with the same principal quantum number and the same l value belong to the same subshell. Shell refers to an energy level in an atom containing electrons, while an orbital is a region. Is An Orbital The Same As A Shell.
From socratic.org
How do you represent electron orbitals through drawings? Socratic Is An Orbital The Same As A Shell Subshell is the pathway in which an electron. H, he +, li +2, etc.) the energy of each orbital within a particular shell is identical. Within a shell (same n n), all. Orbitals with the same principal quantum number and the same l value belong to the same subshell. Each atomic orbital can be occupied by a maximum of two. Is An Orbital The Same As A Shell.
From www.britannica.com
Sorbital physics Britannica Is An Orbital The Same As A Shell Shell refers to an energy level in an atom containing electrons, while an orbital is a region within a shell where electrons are likely to be found. Review the types of orbitals (s, p, d, f) and their. Orbitals exist at specific energy levels and electrons can only be found at these specific levels, not in between them. All electrons. Is An Orbital The Same As A Shell.
From www.youtube.com
What are shells,subshells and orbitals? Difference between shells Is An Orbital The Same As A Shell Learn about orbitals, the three dimensional descriptions of electron locations around atoms, and how they combine to form bonds. Within a shell (same n n), all. All electrons that have the same value for n n (the principle quantum number) are in the same shell. H, he +, li +2, etc.) the energy of each orbital within a particular shell. Is An Orbital The Same As A Shell.
From chemistryskills.com
Shapes of Orbitals and their Types Chemistry Skills Is An Orbital The Same As A Shell Subshell is the pathway in which an electron. Review the types of orbitals (s, p, d, f) and their. H, he +, li +2, etc.) the energy of each orbital within a particular shell is identical. Orbitals exist at specific energy levels and electrons can only be found at these specific levels, not in between them. Shell refers to an. Is An Orbital The Same As A Shell.
From www.youtube.com
Shells, Subshells, and Orbitals l Understand the difference YouTube Is An Orbital The Same As A Shell Review the types of orbitals (s, p, d, f) and their. H, he +, li +2, etc.) the energy of each orbital within a particular shell is identical. Shell refers to an energy level in an atom containing electrons, while an orbital is a region within a shell where electrons are likely to be found. Shell is the pathway followed. Is An Orbital The Same As A Shell.
From newtondesk.com
Periodic Elements Electron Shells, SubShells, and Orbitals Chemistry Is An Orbital The Same As A Shell Within a shell (same n n), all. Review the types of orbitals (s, p, d, f) and their. Each atomic orbital can be occupied by a maximum of two electrons. Orbitals exist at specific energy levels and electrons can only be found at these specific levels, not in between them. Learn about orbitals, the three dimensional descriptions of electron locations. Is An Orbital The Same As A Shell.
From www.sciencefacts.net
Electron Configuration Definition, Examples, Chart, and Diagram Is An Orbital The Same As A Shell Each atomic orbital can be occupied by a maximum of two electrons. Shell is the pathway followed by electrons around an atom’s nucleus. All electrons that have the same value for n n (the principle quantum number) are in the same shell. H, he +, li +2, etc.) the energy of each orbital within a particular shell is identical. Within. Is An Orbital The Same As A Shell.
From gzscienceclassonline.weebly.com
1. Electron Configuration Is An Orbital The Same As A Shell Orbitals with the same principal quantum number and the same l value belong to the same subshell. Review the types of orbitals (s, p, d, f) and their. Shell is the pathway followed by electrons around an atom’s nucleus. Within a shell (same n n), all. Learn about orbitals, the three dimensional descriptions of electron locations around atoms, and how. Is An Orbital The Same As A Shell.
From socratic.org
What is the difference between electron shells and electron orbitals Is An Orbital The Same As A Shell All electrons that have the same value for n n (the principle quantum number) are in the same shell. Orbitals with the same principal quantum number and the same l value belong to the same subshell. Subshell is the pathway in which an electron. Review the types of orbitals (s, p, d, f) and their. Shell refers to an energy. Is An Orbital The Same As A Shell.
From pediaa.com
Difference Between Shell Subshell and Orbital Definition, Structure Is An Orbital The Same As A Shell Subshell is the pathway in which an electron. H, he +, li +2, etc.) the energy of each orbital within a particular shell is identical. Shell refers to an energy level in an atom containing electrons, while an orbital is a region within a shell where electrons are likely to be found. All electrons that have the same value for. Is An Orbital The Same As A Shell.
From www.youtube.com
When the electrons are successively filled in the orbitals of same Is An Orbital The Same As A Shell H, he +, li +2, etc.) the energy of each orbital within a particular shell is identical. Review the types of orbitals (s, p, d, f) and their. Subshell is the pathway in which an electron. Orbitals exist at specific energy levels and electrons can only be found at these specific levels, not in between them. Within a shell (same. Is An Orbital The Same As A Shell.
From www.youtube.com
Orbits, Shells, Subshells and Orbitals CSIR Life Sciences CYTOLOGY Is An Orbital The Same As A Shell Subshell is the pathway in which an electron. Within a shell (same n n), all. Orbitals with the same principal quantum number and the same l value belong to the same subshell. All electrons that have the same value for n n (the principle quantum number) are in the same shell. Review the types of orbitals (s, p, d, f). Is An Orbital The Same As A Shell.
From www.slideserve.com
PPT Shells and Subshells PowerPoint Presentation, free download ID Is An Orbital The Same As A Shell Shell is the pathway followed by electrons around an atom’s nucleus. Orbitals exist at specific energy levels and electrons can only be found at these specific levels, not in between them. Within a shell (same n n), all. Each atomic orbital can be occupied by a maximum of two electrons. Subshell is the pathway in which an electron. All electrons. Is An Orbital The Same As A Shell.
From www.slideserve.com
PPT 1. Structure and Bonding PowerPoint Presentation, free download Is An Orbital The Same As A Shell Subshell is the pathway in which an electron. Each atomic orbital can be occupied by a maximum of two electrons. H, he +, li +2, etc.) the energy of each orbital within a particular shell is identical. Shell is the pathway followed by electrons around an atom’s nucleus. Shell refers to an energy level in an atom containing electrons, while. Is An Orbital The Same As A Shell.
From www.britannica.com
Electron shell Definition & Facts Britannica Is An Orbital The Same As A Shell Subshell is the pathway in which an electron. Within a shell (same n n), all. Shell refers to an energy level in an atom containing electrons, while an orbital is a region within a shell where electrons are likely to be found. H, he +, li +2, etc.) the energy of each orbital within a particular shell is identical. Each. Is An Orbital The Same As A Shell.
From www.chemistrystudent.com
Electron Orbitals (ALevel) ChemistryStudent Is An Orbital The Same As A Shell Orbitals exist at specific energy levels and electrons can only be found at these specific levels, not in between them. Subshell is the pathway in which an electron. Each atomic orbital can be occupied by a maximum of two electrons. Within a shell (same n n), all. H, he +, li +2, etc.) the energy of each orbital within a. Is An Orbital The Same As A Shell.
From general.chemistrysteps.com
Orbital Diagrams Chemistry Steps Is An Orbital The Same As A Shell Each atomic orbital can be occupied by a maximum of two electrons. Within a shell (same n n), all. All electrons that have the same value for n n (the principle quantum number) are in the same shell. H, he +, li +2, etc.) the energy of each orbital within a particular shell is identical. Learn about orbitals, the three. Is An Orbital The Same As A Shell.
From www.coscinecreative.com
What is the Difference in a Shell, Subshell and Orbital? — CoScine Creative Is An Orbital The Same As A Shell Learn about orbitals, the three dimensional descriptions of electron locations around atoms, and how they combine to form bonds. Shell refers to an energy level in an atom containing electrons, while an orbital is a region within a shell where electrons are likely to be found. Orbitals exist at specific energy levels and electrons can only be found at these. Is An Orbital The Same As A Shell.