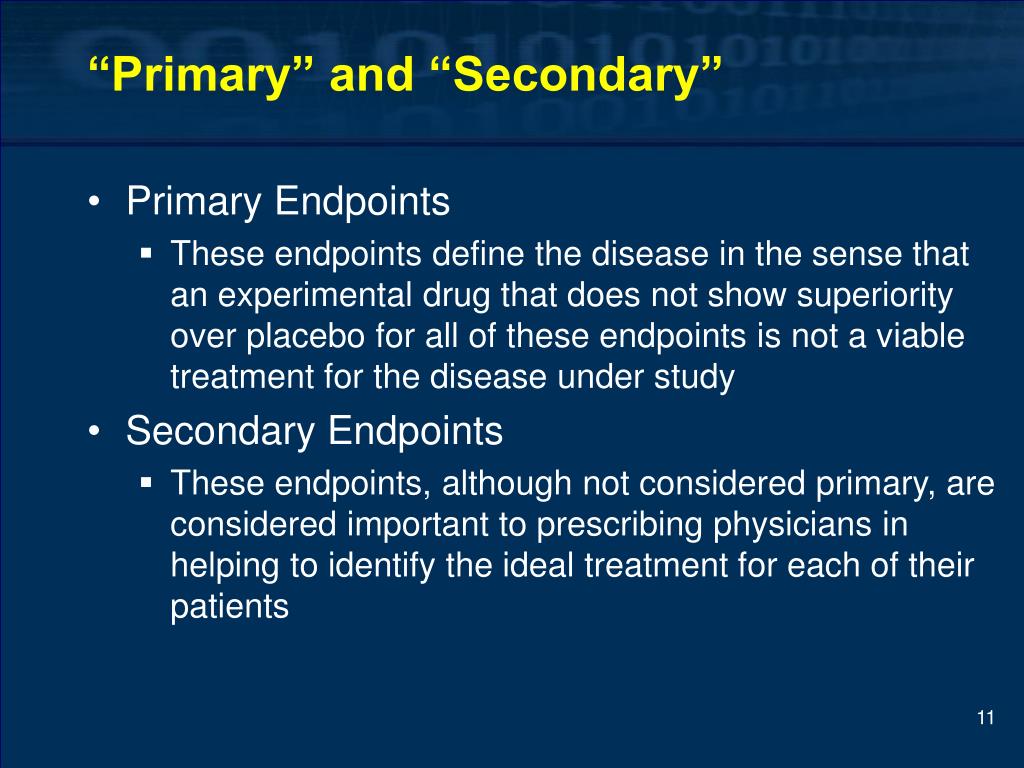
Co Primary Endpoints Definition . Used when a treatment effect on all of two or more clinical features is critically important to demonstrate. When the treatment benefit is still uncertain in the subgroup, sometimes registrational trials are designed to have co. 1) choosing the appropriate outcomes; In other cases, an effect on any of several. A clinical variable (outcome, symptom, or surrogate) which reflects the condition of a disease. The purpose of this guidance is to describe various strategies for grouping and ordering endpoints for analysis and applying. The disease into a single endpoint or effects should be demonstrated on multiple endpoints. 2) defining what will be the primary and what will be secondary endpoints; There are several key aspects regarding primary and secondary outcomes:
from www.slideserve.com
The purpose of this guidance is to describe various strategies for grouping and ordering endpoints for analysis and applying. 1) choosing the appropriate outcomes; A clinical variable (outcome, symptom, or surrogate) which reflects the condition of a disease. 2) defining what will be the primary and what will be secondary endpoints; The disease into a single endpoint or effects should be demonstrated on multiple endpoints. When the treatment benefit is still uncertain in the subgroup, sometimes registrational trials are designed to have co. There are several key aspects regarding primary and secondary outcomes: In other cases, an effect on any of several. Used when a treatment effect on all of two or more clinical features is critically important to demonstrate.
PPT Multiple Primary Endpoints in Clinical Trials PowerPoint
Co Primary Endpoints Definition The purpose of this guidance is to describe various strategies for grouping and ordering endpoints for analysis and applying. There are several key aspects regarding primary and secondary outcomes: The disease into a single endpoint or effects should be demonstrated on multiple endpoints. The purpose of this guidance is to describe various strategies for grouping and ordering endpoints for analysis and applying. Used when a treatment effect on all of two or more clinical features is critically important to demonstrate. When the treatment benefit is still uncertain in the subgroup, sometimes registrational trials are designed to have co. A clinical variable (outcome, symptom, or surrogate) which reflects the condition of a disease. 2) defining what will be the primary and what will be secondary endpoints; In other cases, an effect on any of several. 1) choosing the appropriate outcomes;
From www.slideserve.com
PPT Assessing Response and Progression in Ovarian Cancer Clinical Co Primary Endpoints Definition When the treatment benefit is still uncertain in the subgroup, sometimes registrational trials are designed to have co. 1) choosing the appropriate outcomes; 2) defining what will be the primary and what will be secondary endpoints; There are several key aspects regarding primary and secondary outcomes: The disease into a single endpoint or effects should be demonstrated on multiple endpoints.. Co Primary Endpoints Definition.
From www.slideserve.com
PPT Hisao Ogawa 1 , Shokei KimMitsuyama 2 , Tomio Jinnouchi 3 Co Primary Endpoints Definition There are several key aspects regarding primary and secondary outcomes: In other cases, an effect on any of several. 2) defining what will be the primary and what will be secondary endpoints; Used when a treatment effect on all of two or more clinical features is critically important to demonstrate. 1) choosing the appropriate outcomes; A clinical variable (outcome, symptom,. Co Primary Endpoints Definition.
From www.slideserve.com
PPT The Role of in Safety Assessment PowerPoint Co Primary Endpoints Definition The disease into a single endpoint or effects should be demonstrated on multiple endpoints. 1) choosing the appropriate outcomes; 2) defining what will be the primary and what will be secondary endpoints; There are several key aspects regarding primary and secondary outcomes: In other cases, an effect on any of several. A clinical variable (outcome, symptom, or surrogate) which reflects. Co Primary Endpoints Definition.
From www.slideserve.com
PPT Transcatheter Aortic Valve Implantation in Inoperable Patients Co Primary Endpoints Definition Used when a treatment effect on all of two or more clinical features is critically important to demonstrate. When the treatment benefit is still uncertain in the subgroup, sometimes registrational trials are designed to have co. 1) choosing the appropriate outcomes; In other cases, an effect on any of several. A clinical variable (outcome, symptom, or surrogate) which reflects the. Co Primary Endpoints Definition.
From slideplayer.com
ACT II The Second UK Phase III Anal Cancer Trial ppt download Co Primary Endpoints Definition The purpose of this guidance is to describe various strategies for grouping and ordering endpoints for analysis and applying. In other cases, an effect on any of several. When the treatment benefit is still uncertain in the subgroup, sometimes registrational trials are designed to have co. Used when a treatment effect on all of two or more clinical features is. Co Primary Endpoints Definition.
From www.slideserve.com
PPT Multiple Primary Endpoints in Clinical Trials PowerPoint Co Primary Endpoints Definition There are several key aspects regarding primary and secondary outcomes: 2) defining what will be the primary and what will be secondary endpoints; The disease into a single endpoint or effects should be demonstrated on multiple endpoints. When the treatment benefit is still uncertain in the subgroup, sometimes registrational trials are designed to have co. Used when a treatment effect. Co Primary Endpoints Definition.
From discourse.datamethods.org
Deviation in primary endpoint definition data analysis Datamethods Co Primary Endpoints Definition The disease into a single endpoint or effects should be demonstrated on multiple endpoints. The purpose of this guidance is to describe various strategies for grouping and ordering endpoints for analysis and applying. 1) choosing the appropriate outcomes; When the treatment benefit is still uncertain in the subgroup, sometimes registrational trials are designed to have co. In other cases, an. Co Primary Endpoints Definition.
From www.researchgate.net
Study endpoints and definitions Study endpoints Primary endpoint Co Primary Endpoints Definition A clinical variable (outcome, symptom, or surrogate) which reflects the condition of a disease. 1) choosing the appropriate outcomes; The disease into a single endpoint or effects should be demonstrated on multiple endpoints. In other cases, an effect on any of several. There are several key aspects regarding primary and secondary outcomes: When the treatment benefit is still uncertain in. Co Primary Endpoints Definition.
From www.researchgate.net
shows the study flow chart and visualizes the primary endpoint Co Primary Endpoints Definition The purpose of this guidance is to describe various strategies for grouping and ordering endpoints for analysis and applying. In other cases, an effect on any of several. 1) choosing the appropriate outcomes; The disease into a single endpoint or effects should be demonstrated on multiple endpoints. Used when a treatment effect on all of two or more clinical features. Co Primary Endpoints Definition.
From www.slideserve.com
PPT Fundamentals of Clinical Trials Endpoints Betsy Garofalo MD Co Primary Endpoints Definition When the treatment benefit is still uncertain in the subgroup, sometimes registrational trials are designed to have co. In other cases, an effect on any of several. 2) defining what will be the primary and what will be secondary endpoints; There are several key aspects regarding primary and secondary outcomes: The disease into a single endpoint or effects should be. Co Primary Endpoints Definition.
From slidetodoc.com
CO1 Reherniation and Reoperation in Lumbar Discectomy Impact Co Primary Endpoints Definition The purpose of this guidance is to describe various strategies for grouping and ordering endpoints for analysis and applying. 2) defining what will be the primary and what will be secondary endpoints; The disease into a single endpoint or effects should be demonstrated on multiple endpoints. In other cases, an effect on any of several. A clinical variable (outcome, symptom,. Co Primary Endpoints Definition.
From www.likelihooo.com
A Discussion of Multiple Testing in Group Sequential Trial D4191C00004 Co Primary Endpoints Definition The purpose of this guidance is to describe various strategies for grouping and ordering endpoints for analysis and applying. Used when a treatment effect on all of two or more clinical features is critically important to demonstrate. When the treatment benefit is still uncertain in the subgroup, sometimes registrational trials are designed to have co. 1) choosing the appropriate outcomes;. Co Primary Endpoints Definition.
From www.researchgate.net
Results for the coprimary endpoints for trastuzumab s. c. vs. i. v. in Co Primary Endpoints Definition A clinical variable (outcome, symptom, or surrogate) which reflects the condition of a disease. When the treatment benefit is still uncertain in the subgroup, sometimes registrational trials are designed to have co. Used when a treatment effect on all of two or more clinical features is critically important to demonstrate. 2) defining what will be the primary and what will. Co Primary Endpoints Definition.
From www.vrogue.co
Endpoint Security Definition Features Benefits And Mo vrogue.co Co Primary Endpoints Definition 2) defining what will be the primary and what will be secondary endpoints; In other cases, an effect on any of several. The purpose of this guidance is to describe various strategies for grouping and ordering endpoints for analysis and applying. When the treatment benefit is still uncertain in the subgroup, sometimes registrational trials are designed to have co. There. Co Primary Endpoints Definition.
From www.slideserve.com
PPT What are Important Endpoints in Anaesthesia Research? PowerPoint Co Primary Endpoints Definition The purpose of this guidance is to describe various strategies for grouping and ordering endpoints for analysis and applying. 1) choosing the appropriate outcomes; A clinical variable (outcome, symptom, or surrogate) which reflects the condition of a disease. 2) defining what will be the primary and what will be secondary endpoints; The disease into a single endpoint or effects should. Co Primary Endpoints Definition.
From cra-school.com
The Endpoint Selection a Complex Process in the Clinical Trials Design Co Primary Endpoints Definition The purpose of this guidance is to describe various strategies for grouping and ordering endpoints for analysis and applying. In other cases, an effect on any of several. 1) choosing the appropriate outcomes; Used when a treatment effect on all of two or more clinical features is critically important to demonstrate. A clinical variable (outcome, symptom, or surrogate) which reflects. Co Primary Endpoints Definition.
From www.slideserve.com
PPT Expanded Indications and Claims for Guidant CRTD Devices Co Primary Endpoints Definition In other cases, an effect on any of several. There are several key aspects regarding primary and secondary outcomes: Used when a treatment effect on all of two or more clinical features is critically important to demonstrate. 1) choosing the appropriate outcomes; The purpose of this guidance is to describe various strategies for grouping and ordering endpoints for analysis and. Co Primary Endpoints Definition.
From www.researchgate.net
of coprimary endpoints and other assessments between patients with and Co Primary Endpoints Definition In other cases, an effect on any of several. Used when a treatment effect on all of two or more clinical features is critically important to demonstrate. 1) choosing the appropriate outcomes; The disease into a single endpoint or effects should be demonstrated on multiple endpoints. 2) defining what will be the primary and what will be secondary endpoints; There. Co Primary Endpoints Definition.
From www.researchgate.net
Coprimary efficacy endpoints over 7 Days. Panel A shows Average Pain Co Primary Endpoints Definition The disease into a single endpoint or effects should be demonstrated on multiple endpoints. The purpose of this guidance is to describe various strategies for grouping and ordering endpoints for analysis and applying. A clinical variable (outcome, symptom, or surrogate) which reflects the condition of a disease. Used when a treatment effect on all of two or more clinical features. Co Primary Endpoints Definition.
From longoncologie.nl
GCT104604 Longoncologie.nl Co Primary Endpoints Definition The purpose of this guidance is to describe various strategies for grouping and ordering endpoints for analysis and applying. There are several key aspects regarding primary and secondary outcomes: The disease into a single endpoint or effects should be demonstrated on multiple endpoints. A clinical variable (outcome, symptom, or surrogate) which reflects the condition of a disease. 2) defining what. Co Primary Endpoints Definition.
From onbiostatistics.blogspot.com
On Biostatistics and Clinical Trials Control for Type I Error (or Co Primary Endpoints Definition 2) defining what will be the primary and what will be secondary endpoints; 1) choosing the appropriate outcomes; A clinical variable (outcome, symptom, or surrogate) which reflects the condition of a disease. The disease into a single endpoint or effects should be demonstrated on multiple endpoints. When the treatment benefit is still uncertain in the subgroup, sometimes registrational trials are. Co Primary Endpoints Definition.
From www.slideserve.com
PPT Multiple Primary Endpoints in Clinical Trials PowerPoint Co Primary Endpoints Definition 2) defining what will be the primary and what will be secondary endpoints; 1) choosing the appropriate outcomes; The disease into a single endpoint or effects should be demonstrated on multiple endpoints. There are several key aspects regarding primary and secondary outcomes: When the treatment benefit is still uncertain in the subgroup, sometimes registrational trials are designed to have co.. Co Primary Endpoints Definition.
From twitter.com
Eric Topol on Twitter "Many clinical trials have "coprimary endpoints Co Primary Endpoints Definition The purpose of this guidance is to describe various strategies for grouping and ordering endpoints for analysis and applying. Used when a treatment effect on all of two or more clinical features is critically important to demonstrate. 1) choosing the appropriate outcomes; The disease into a single endpoint or effects should be demonstrated on multiple endpoints. 2) defining what will. Co Primary Endpoints Definition.
From onbiostatistics.blogspot.com
On Biostatistics and Clinical Trials December 2016 Co Primary Endpoints Definition 2) defining what will be the primary and what will be secondary endpoints; The purpose of this guidance is to describe various strategies for grouping and ordering endpoints for analysis and applying. Used when a treatment effect on all of two or more clinical features is critically important to demonstrate. There are several key aspects regarding primary and secondary outcomes:. Co Primary Endpoints Definition.
From www.slideserve.com
PPT Fundamentals of Clinical Trials Endpoints Betsy Garofalo MD Co Primary Endpoints Definition There are several key aspects regarding primary and secondary outcomes: Used when a treatment effect on all of two or more clinical features is critically important to demonstrate. The purpose of this guidance is to describe various strategies for grouping and ordering endpoints for analysis and applying. When the treatment benefit is still uncertain in the subgroup, sometimes registrational trials. Co Primary Endpoints Definition.
From www.researchgate.net
Study screening process and definition of the primary endpoint Co Primary Endpoints Definition In other cases, an effect on any of several. The disease into a single endpoint or effects should be demonstrated on multiple endpoints. There are several key aspects regarding primary and secondary outcomes: When the treatment benefit is still uncertain in the subgroup, sometimes registrational trials are designed to have co. The purpose of this guidance is to describe various. Co Primary Endpoints Definition.
From slideplayer.com
TCT 2016, Washington convention center ppt download Co Primary Endpoints Definition The disease into a single endpoint or effects should be demonstrated on multiple endpoints. When the treatment benefit is still uncertain in the subgroup, sometimes registrational trials are designed to have co. In other cases, an effect on any of several. 2) defining what will be the primary and what will be secondary endpoints; A clinical variable (outcome, symptom, or. Co Primary Endpoints Definition.
From www.researchgate.net
Primary and secondary endpoints Download Table Co Primary Endpoints Definition A clinical variable (outcome, symptom, or surrogate) which reflects the condition of a disease. The disease into a single endpoint or effects should be demonstrated on multiple endpoints. The purpose of this guidance is to describe various strategies for grouping and ordering endpoints for analysis and applying. Used when a treatment effect on all of two or more clinical features. Co Primary Endpoints Definition.
From www.slideserve.com
PPT Diuretic Optimization Strategies Evaluation in Acute Heart Co Primary Endpoints Definition A clinical variable (outcome, symptom, or surrogate) which reflects the condition of a disease. In other cases, an effect on any of several. 1) choosing the appropriate outcomes; Used when a treatment effect on all of two or more clinical features is critically important to demonstrate. The disease into a single endpoint or effects should be demonstrated on multiple endpoints.. Co Primary Endpoints Definition.
From www.cureus.com
Research Question, Objectives, and Endpoints in Clinical and Co Primary Endpoints Definition 1) choosing the appropriate outcomes; A clinical variable (outcome, symptom, or surrogate) which reflects the condition of a disease. There are several key aspects regarding primary and secondary outcomes: The disease into a single endpoint or effects should be demonstrated on multiple endpoints. 2) defining what will be the primary and what will be secondary endpoints; When the treatment benefit. Co Primary Endpoints Definition.
From slideplayer.com
TCT 2016, Washington convention center ppt download Co Primary Endpoints Definition Used when a treatment effect on all of two or more clinical features is critically important to demonstrate. There are several key aspects regarding primary and secondary outcomes: 2) defining what will be the primary and what will be secondary endpoints; The purpose of this guidance is to describe various strategies for grouping and ordering endpoints for analysis and applying.. Co Primary Endpoints Definition.
From www.ciscrp.org
Endpoint Selection in Plain Language Summaries Insights from CISCRP’s Co Primary Endpoints Definition A clinical variable (outcome, symptom, or surrogate) which reflects the condition of a disease. 2) defining what will be the primary and what will be secondary endpoints; The purpose of this guidance is to describe various strategies for grouping and ordering endpoints for analysis and applying. In other cases, an effect on any of several. The disease into a single. Co Primary Endpoints Definition.
From www.slideserve.com
PPT What are Important Endpoints in Anaesthesia Research? PowerPoint Co Primary Endpoints Definition A clinical variable (outcome, symptom, or surrogate) which reflects the condition of a disease. The purpose of this guidance is to describe various strategies for grouping and ordering endpoints for analysis and applying. There are several key aspects regarding primary and secondary outcomes: 2) defining what will be the primary and what will be secondary endpoints; The disease into a. Co Primary Endpoints Definition.
From www.researchgate.net
Definitions of primary and secondary endpoints. SSIs were diagnosed Co Primary Endpoints Definition In other cases, an effect on any of several. Used when a treatment effect on all of two or more clinical features is critically important to demonstrate. When the treatment benefit is still uncertain in the subgroup, sometimes registrational trials are designed to have co. There are several key aspects regarding primary and secondary outcomes: The disease into a single. Co Primary Endpoints Definition.
From www.tldrpharmacy.com
A Guide to Clinical Trial Endpoints — tl;dr pharmacy Co Primary Endpoints Definition A clinical variable (outcome, symptom, or surrogate) which reflects the condition of a disease. The purpose of this guidance is to describe various strategies for grouping and ordering endpoints for analysis and applying. When the treatment benefit is still uncertain in the subgroup, sometimes registrational trials are designed to have co. There are several key aspects regarding primary and secondary. Co Primary Endpoints Definition.