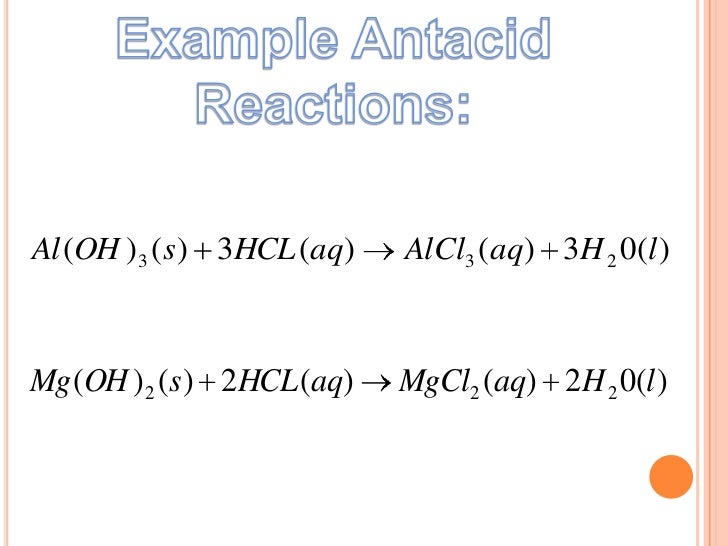
Antacid Chemical Reaction Word Equation . In this experiment, you will standardize a solution of base using the analytical technique known as titration. The balanced chemical equation for the reaction of the main ingredient of the antacid tablet (caco 3) with the hcl is as follows. They don't need to raise the stomach acid ph all the way to a neutral ph of 7, but just raising the ph to 3 or 4 will make a person feel better. 2 hcl (aq) + caco. Other antacids, such as alka seltzer, work in similar ways. Below, show the mechanism and products for reaction in which. Antacids essentially work by increasing the ph of the very acidic stomach acid. The following table contains a list of the active ingredients found in several common commercial antacids, and the reactions by which these. Mix milk of magnesia (mom) with universal indicator and observe the dramatic rainbow of colors as the antacid dissolves in. Write balanced chemical equations for neutralization reactions and determine if the resulting solution will be acidic, basic, or neutral. This is done through an acid/base chemical reaction, which is shown in equation 1 below. Antacids are compounds that neutralize stomach acid.
from www.slideshare.net
Write balanced chemical equations for neutralization reactions and determine if the resulting solution will be acidic, basic, or neutral. They don't need to raise the stomach acid ph all the way to a neutral ph of 7, but just raising the ph to 3 or 4 will make a person feel better. Below, show the mechanism and products for reaction in which. Antacids are compounds that neutralize stomach acid. The balanced chemical equation for the reaction of the main ingredient of the antacid tablet (caco 3) with the hcl is as follows. 2 hcl (aq) + caco. Mix milk of magnesia (mom) with universal indicator and observe the dramatic rainbow of colors as the antacid dissolves in. Other antacids, such as alka seltzer, work in similar ways. The following table contains a list of the active ingredients found in several common commercial antacids, and the reactions by which these. Antacids essentially work by increasing the ph of the very acidic stomach acid.
Antacids
Antacid Chemical Reaction Word Equation 2 hcl (aq) + caco. This is done through an acid/base chemical reaction, which is shown in equation 1 below. 2 hcl (aq) + caco. The balanced chemical equation for the reaction of the main ingredient of the antacid tablet (caco 3) with the hcl is as follows. They don't need to raise the stomach acid ph all the way to a neutral ph of 7, but just raising the ph to 3 or 4 will make a person feel better. The following table contains a list of the active ingredients found in several common commercial antacids, and the reactions by which these. Write balanced chemical equations for neutralization reactions and determine if the resulting solution will be acidic, basic, or neutral. Other antacids, such as alka seltzer, work in similar ways. Mix milk of magnesia (mom) with universal indicator and observe the dramatic rainbow of colors as the antacid dissolves in. Antacids essentially work by increasing the ph of the very acidic stomach acid. Antacids are compounds that neutralize stomach acid. Below, show the mechanism and products for reaction in which. In this experiment, you will standardize a solution of base using the analytical technique known as titration.
From askfilo.com
(e) Antacid (d) Antiseptic 5. Write word equations and then balanced equa.. Antacid Chemical Reaction Word Equation The balanced chemical equation for the reaction of the main ingredient of the antacid tablet (caco 3) with the hcl is as follows. Other antacids, such as alka seltzer, work in similar ways. The following table contains a list of the active ingredients found in several common commercial antacids, and the reactions by which these. Antacids are compounds that neutralize. Antacid Chemical Reaction Word Equation.
From study.com
Writing Balanced Chemical Reactions Video & Lesson Transcript Antacid Chemical Reaction Word Equation Mix milk of magnesia (mom) with universal indicator and observe the dramatic rainbow of colors as the antacid dissolves in. Below, show the mechanism and products for reaction in which. They don't need to raise the stomach acid ph all the way to a neutral ph of 7, but just raising the ph to 3 or 4 will make a. Antacid Chemical Reaction Word Equation.
From www.slideserve.com
PPT Antacids PowerPoint Presentation ID1994845 Antacid Chemical Reaction Word Equation Below, show the mechanism and products for reaction in which. Mix milk of magnesia (mom) with universal indicator and observe the dramatic rainbow of colors as the antacid dissolves in. Antacids are compounds that neutralize stomach acid. Antacids essentially work by increasing the ph of the very acidic stomach acid. Other antacids, such as alka seltzer, work in similar ways.. Antacid Chemical Reaction Word Equation.
From www.slideshare.net
Antacids Antacid Chemical Reaction Word Equation This is done through an acid/base chemical reaction, which is shown in equation 1 below. 2 hcl (aq) + caco. In this experiment, you will standardize a solution of base using the analytical technique known as titration. Other antacids, such as alka seltzer, work in similar ways. Write balanced chemical equations for neutralization reactions and determine if the resulting solution. Antacid Chemical Reaction Word Equation.
From lessonlibrarysnotter.z13.web.core.windows.net
Identify The Parts Of A Chemical Equation Antacid Chemical Reaction Word Equation 2 hcl (aq) + caco. The balanced chemical equation for the reaction of the main ingredient of the antacid tablet (caco 3) with the hcl is as follows. Antacids are compounds that neutralize stomach acid. Below, show the mechanism and products for reaction in which. Antacids essentially work by increasing the ph of the very acidic stomach acid. Mix milk. Antacid Chemical Reaction Word Equation.
From www.numerade.com
SOLVEDWrite the balanced chemical equation for the neutralization Antacid Chemical Reaction Word Equation 2 hcl (aq) + caco. They don't need to raise the stomach acid ph all the way to a neutral ph of 7, but just raising the ph to 3 or 4 will make a person feel better. Antacids are compounds that neutralize stomach acid. Mix milk of magnesia (mom) with universal indicator and observe the dramatic rainbow of colors. Antacid Chemical Reaction Word Equation.
From www.numerade.com
SOLVED The table below lists several common antacid brands, their Antacid Chemical Reaction Word Equation The balanced chemical equation for the reaction of the main ingredient of the antacid tablet (caco 3) with the hcl is as follows. They don't need to raise the stomach acid ph all the way to a neutral ph of 7, but just raising the ph to 3 or 4 will make a person feel better. Write balanced chemical equations. Antacid Chemical Reaction Word Equation.
From slideplayer.com
Chapter 2 Antacids. ppt download Antacid Chemical Reaction Word Equation Antacids are compounds that neutralize stomach acid. The balanced chemical equation for the reaction of the main ingredient of the antacid tablet (caco 3) with the hcl is as follows. This is done through an acid/base chemical reaction, which is shown in equation 1 below. Antacids essentially work by increasing the ph of the very acidic stomach acid. Write balanced. Antacid Chemical Reaction Word Equation.
From www.slideshare.net
Antacids Antacid Chemical Reaction Word Equation Mix milk of magnesia (mom) with universal indicator and observe the dramatic rainbow of colors as the antacid dissolves in. Other antacids, such as alka seltzer, work in similar ways. The balanced chemical equation for the reaction of the main ingredient of the antacid tablet (caco 3) with the hcl is as follows. They don't need to raise the stomach. Antacid Chemical Reaction Word Equation.
From www.slideserve.com
PPT Chemistry of antacids PowerPoint Presentation, free download ID Antacid Chemical Reaction Word Equation Mix milk of magnesia (mom) with universal indicator and observe the dramatic rainbow of colors as the antacid dissolves in. This is done through an acid/base chemical reaction, which is shown in equation 1 below. Antacids essentially work by increasing the ph of the very acidic stomach acid. 2 hcl (aq) + caco. Below, show the mechanism and products for. Antacid Chemical Reaction Word Equation.
From www.numerade.com
Antacids are compounds that neutralize stomach ac… Antacid Chemical Reaction Word Equation The following table contains a list of the active ingredients found in several common commercial antacids, and the reactions by which these. Write balanced chemical equations for neutralization reactions and determine if the resulting solution will be acidic, basic, or neutral. Antacids are compounds that neutralize stomach acid. Antacids essentially work by increasing the ph of the very acidic stomach. Antacid Chemical Reaction Word Equation.
From www.slideserve.com
PPT Antacids PowerPoint Presentation ID1994845 Antacid Chemical Reaction Word Equation This is done through an acid/base chemical reaction, which is shown in equation 1 below. Antacids essentially work by increasing the ph of the very acidic stomach acid. The following table contains a list of the active ingredients found in several common commercial antacids, and the reactions by which these. The balanced chemical equation for the reaction of the main. Antacid Chemical Reaction Word Equation.
From www.youtube.com
D.4 Antacids (SL) YouTube Antacid Chemical Reaction Word Equation This is done through an acid/base chemical reaction, which is shown in equation 1 below. In this experiment, you will standardize a solution of base using the analytical technique known as titration. Below, show the mechanism and products for reaction in which. Antacids are compounds that neutralize stomach acid. The following table contains a list of the active ingredients found. Antacid Chemical Reaction Word Equation.
From www.slideserve.com
PPT If we ’ re going to do chemical reactions with antacids, we ’ d Antacid Chemical Reaction Word Equation Antacids are compounds that neutralize stomach acid. Write balanced chemical equations for neutralization reactions and determine if the resulting solution will be acidic, basic, or neutral. Antacids essentially work by increasing the ph of the very acidic stomach acid. Other antacids, such as alka seltzer, work in similar ways. In this experiment, you will standardize a solution of base using. Antacid Chemical Reaction Word Equation.
From brainly.com
AlkaSeltzer® is an antacid. One of its active ingredients is NAHCO3 Antacid Chemical Reaction Word Equation The following table contains a list of the active ingredients found in several common commercial antacids, and the reactions by which these. Write balanced chemical equations for neutralization reactions and determine if the resulting solution will be acidic, basic, or neutral. 2 hcl (aq) + caco. Antacids are compounds that neutralize stomach acid. They don't need to raise the stomach. Antacid Chemical Reaction Word Equation.
From byjus.com
Write the neutralization reaction between Hydrochloric acid HCI and Antacid Chemical Reaction Word Equation Antacids essentially work by increasing the ph of the very acidic stomach acid. Below, show the mechanism and products for reaction in which. The following table contains a list of the active ingredients found in several common commercial antacids, and the reactions by which these. They don't need to raise the stomach acid ph all the way to a neutral. Antacid Chemical Reaction Word Equation.
From www.slideserve.com
PPT Antacids PowerPoint Presentation, free download ID3824036 Antacid Chemical Reaction Word Equation The balanced chemical equation for the reaction of the main ingredient of the antacid tablet (caco 3) with the hcl is as follows. In this experiment, you will standardize a solution of base using the analytical technique known as titration. 2 hcl (aq) + caco. The following table contains a list of the active ingredients found in several common commercial. Antacid Chemical Reaction Word Equation.
From www.numerade.com
SOLVED Write a balanced chemical equation for the neutralization Antacid Chemical Reaction Word Equation Mix milk of magnesia (mom) with universal indicator and observe the dramatic rainbow of colors as the antacid dissolves in. The balanced chemical equation for the reaction of the main ingredient of the antacid tablet (caco 3) with the hcl is as follows. Antacids are compounds that neutralize stomach acid. Below, show the mechanism and products for reaction in which.. Antacid Chemical Reaction Word Equation.
From www.slideserve.com
PPT If we ’ re going to do chemical reactions with antacids, we ’ d Antacid Chemical Reaction Word Equation They don't need to raise the stomach acid ph all the way to a neutral ph of 7, but just raising the ph to 3 or 4 will make a person feel better. Below, show the mechanism and products for reaction in which. This is done through an acid/base chemical reaction, which is shown in equation 1 below. Other antacids,. Antacid Chemical Reaction Word Equation.
From www.slideserve.com
PPT If we ’ re going to do chemical reactions with antacids, we ’ d Antacid Chemical Reaction Word Equation They don't need to raise the stomach acid ph all the way to a neutral ph of 7, but just raising the ph to 3 or 4 will make a person feel better. 2 hcl (aq) + caco. Other antacids, such as alka seltzer, work in similar ways. Below, show the mechanism and products for reaction in which. The balanced. Antacid Chemical Reaction Word Equation.
From slideplayer.com
Chapter 2 Antacids. ppt download Antacid Chemical Reaction Word Equation They don't need to raise the stomach acid ph all the way to a neutral ph of 7, but just raising the ph to 3 or 4 will make a person feel better. The balanced chemical equation for the reaction of the main ingredient of the antacid tablet (caco 3) with the hcl is as follows. In this experiment, you. Antacid Chemical Reaction Word Equation.
From study.com
Neutralization Reaction Definition, Equation & Examples Lesson Antacid Chemical Reaction Word Equation The balanced chemical equation for the reaction of the main ingredient of the antacid tablet (caco 3) with the hcl is as follows. Antacids essentially work by increasing the ph of the very acidic stomach acid. 2 hcl (aq) + caco. The following table contains a list of the active ingredients found in several common commercial antacids, and the reactions. Antacid Chemical Reaction Word Equation.
From www.slideserve.com
PPT Drugs used in the treatment of peptic ulcer PowerPoint Antacid Chemical Reaction Word Equation The balanced chemical equation for the reaction of the main ingredient of the antacid tablet (caco 3) with the hcl is as follows. Mix milk of magnesia (mom) with universal indicator and observe the dramatic rainbow of colors as the antacid dissolves in. The following table contains a list of the active ingredients found in several common commercial antacids, and. Antacid Chemical Reaction Word Equation.
From www.numerade.com
Antacid Fizz When an antacid tablet dissolves in water, the fizz is due Antacid Chemical Reaction Word Equation This is done through an acid/base chemical reaction, which is shown in equation 1 below. In this experiment, you will standardize a solution of base using the analytical technique known as titration. Write balanced chemical equations for neutralization reactions and determine if the resulting solution will be acidic, basic, or neutral. Other antacids, such as alka seltzer, work in similar. Antacid Chemical Reaction Word Equation.
From www.teachoo.com
[Class 7] Neutralisation Reaction Concept, Formula Teachoo Antacid Chemical Reaction Word Equation They don't need to raise the stomach acid ph all the way to a neutral ph of 7, but just raising the ph to 3 or 4 will make a person feel better. Antacids essentially work by increasing the ph of the very acidic stomach acid. This is done through an acid/base chemical reaction, which is shown in equation 1. Antacid Chemical Reaction Word Equation.
From www.slideshare.net
Chemistry investigatory project on antacids Antacid Chemical Reaction Word Equation They don't need to raise the stomach acid ph all the way to a neutral ph of 7, but just raising the ph to 3 or 4 will make a person feel better. The following table contains a list of the active ingredients found in several common commercial antacids, and the reactions by which these. Other antacids, such as alka. Antacid Chemical Reaction Word Equation.
From www.slideserve.com
PPT If we ’ re going to do chemical reactions with antacids, we ’ d Antacid Chemical Reaction Word Equation Write balanced chemical equations for neutralization reactions and determine if the resulting solution will be acidic, basic, or neutral. Below, show the mechanism and products for reaction in which. This is done through an acid/base chemical reaction, which is shown in equation 1 below. Mix milk of magnesia (mom) with universal indicator and observe the dramatic rainbow of colors as. Antacid Chemical Reaction Word Equation.
From www.slideserve.com
PPT If we ’ re going to do chemical reactions with antacids, we ’ d Antacid Chemical Reaction Word Equation Other antacids, such as alka seltzer, work in similar ways. The following table contains a list of the active ingredients found in several common commercial antacids, and the reactions by which these. This is done through an acid/base chemical reaction, which is shown in equation 1 below. Mix milk of magnesia (mom) with universal indicator and observe the dramatic rainbow. Antacid Chemical Reaction Word Equation.
From www.numerade.com
SOLVED Antacids are used to treat acid reflux by neutralizing stomach Antacid Chemical Reaction Word Equation Antacids essentially work by increasing the ph of the very acidic stomach acid. Antacids are compounds that neutralize stomach acid. They don't need to raise the stomach acid ph all the way to a neutral ph of 7, but just raising the ph to 3 or 4 will make a person feel better. Write balanced chemical equations for neutralization reactions. Antacid Chemical Reaction Word Equation.
From www.numerade.com
SOLVED Aluminum hydroxide in antacid tablets neutralize hydrochloric Antacid Chemical Reaction Word Equation 2 hcl (aq) + caco. Below, show the mechanism and products for reaction in which. This is done through an acid/base chemical reaction, which is shown in equation 1 below. Antacids are compounds that neutralize stomach acid. Antacids essentially work by increasing the ph of the very acidic stomach acid. They don't need to raise the stomach acid ph all. Antacid Chemical Reaction Word Equation.
From www.slideserve.com
PPT Chemistry of antacids PowerPoint Presentation, free download ID Antacid Chemical Reaction Word Equation They don't need to raise the stomach acid ph all the way to a neutral ph of 7, but just raising the ph to 3 or 4 will make a person feel better. Other antacids, such as alka seltzer, work in similar ways. In this experiment, you will standardize a solution of base using the analytical technique known as titration.. Antacid Chemical Reaction Word Equation.
From www.toppr.com
A chemical compound 'X' used in soda acid fire extinguishers and also Antacid Chemical Reaction Word Equation 2 hcl (aq) + caco. Write balanced chemical equations for neutralization reactions and determine if the resulting solution will be acidic, basic, or neutral. The following table contains a list of the active ingredients found in several common commercial antacids, and the reactions by which these. Other antacids, such as alka seltzer, work in similar ways. Antacids are compounds that. Antacid Chemical Reaction Word Equation.
From www.wikihow.com
The Best Way to Write a Chemical Equation wikiHow Antacid Chemical Reaction Word Equation Write balanced chemical equations for neutralization reactions and determine if the resulting solution will be acidic, basic, or neutral. The balanced chemical equation for the reaction of the main ingredient of the antacid tablet (caco 3) with the hcl is as follows. Other antacids, such as alka seltzer, work in similar ways. 2 hcl (aq) + caco. Below, show the. Antacid Chemical Reaction Word Equation.
From slideplayer.com
pH regulation in stomach ppt download Antacid Chemical Reaction Word Equation This is done through an acid/base chemical reaction, which is shown in equation 1 below. 2 hcl (aq) + caco. The following table contains a list of the active ingredients found in several common commercial antacids, and the reactions by which these. They don't need to raise the stomach acid ph all the way to a neutral ph of 7,. Antacid Chemical Reaction Word Equation.
From www.numerade.com
SOLVED Part A Write the balanced chemical equation for the Antacid Chemical Reaction Word Equation Other antacids, such as alka seltzer, work in similar ways. This is done through an acid/base chemical reaction, which is shown in equation 1 below. Antacids are compounds that neutralize stomach acid. Below, show the mechanism and products for reaction in which. The balanced chemical equation for the reaction of the main ingredient of the antacid tablet (caco 3) with. Antacid Chemical Reaction Word Equation.