Chlorine Has How Many Bonds . This time two chlorine atoms add to a molecule across the. A chlorine atom has 7 electrons in its outer shell. Chlorine is in group 7 of the. Diatomic molecules such as hydrogen (\(\ce{h2}\)), chlorine (\(\ce{cl2}\)), fluorine (\(\ce{f2}\)), etc. In cl 2 molecule, each chlorine atom. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a. Why does a chlorine molecule have a covalent bond? Chlorine can also react with alkenes via the electrophilic addition mechanism. When two chlorine atoms form a chlorine molecule, they share one pair of electrons.
from chemsimplified.com
This time two chlorine atoms add to a molecule across the. A chlorine atom has 7 electrons in its outer shell. Chlorine can also react with alkenes via the electrophilic addition mechanism. In cl 2 molecule, each chlorine atom. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a. Why does a chlorine molecule have a covalent bond? When two chlorine atoms form a chlorine molecule, they share one pair of electrons. Diatomic molecules such as hydrogen (\(\ce{h2}\)), chlorine (\(\ce{cl2}\)), fluorine (\(\ce{f2}\)), etc. Chlorine is in group 7 of the.
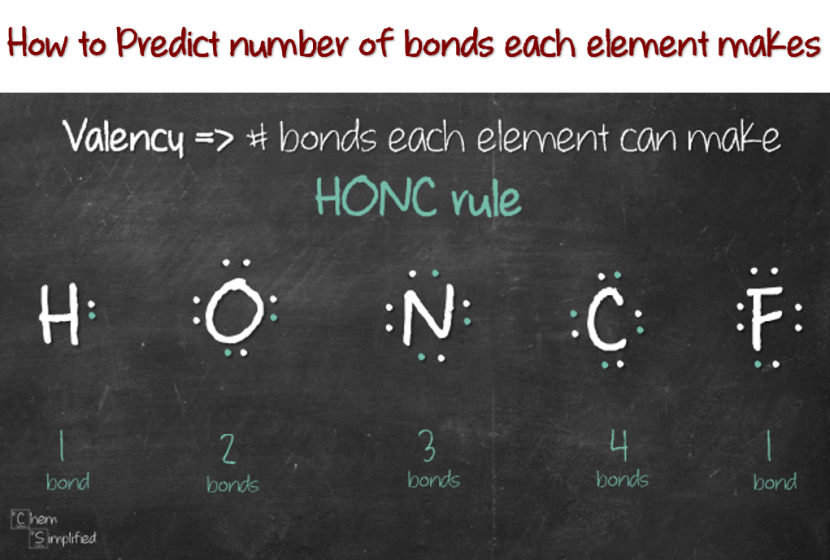
How to Predict number of bonds each element forms ChemSimplified
Chlorine Has How Many Bonds Why does a chlorine molecule have a covalent bond? A chlorine atom has 7 electrons in its outer shell. This time two chlorine atoms add to a molecule across the. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a. Chlorine can also react with alkenes via the electrophilic addition mechanism. Chlorine is in group 7 of the. When two chlorine atoms form a chlorine molecule, they share one pair of electrons. Diatomic molecules such as hydrogen (\(\ce{h2}\)), chlorine (\(\ce{cl2}\)), fluorine (\(\ce{f2}\)), etc. Why does a chlorine molecule have a covalent bond? In cl 2 molecule, each chlorine atom.
From surfguppy.com
What is Ionic Bond Surfguppy Chemistry made easy visual learning Chlorine Has How Many Bonds An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a. Chlorine is in group 7 of the. When two chlorine atoms form a chlorine molecule, they share one pair of electrons. In cl 2 molecule, each. Chlorine Has How Many Bonds.
From slideplayer.com
Chemical Bonds Section ppt download Chlorine Has How Many Bonds When two chlorine atoms form a chlorine molecule, they share one pair of electrons. Why does a chlorine molecule have a covalent bond? Diatomic molecules such as hydrogen (\(\ce{h2}\)), chlorine (\(\ce{cl2}\)), fluorine (\(\ce{f2}\)), etc. This time two chlorine atoms add to a molecule across the. In cl 2 molecule, each chlorine atom. An atom of sodium (na) donates one of. Chlorine Has How Many Bonds.
From www.alamy.com
Sodium Chloride ionic bond formation. NaCl structure. Sodium and Chlorine Has How Many Bonds Chlorine can also react with alkenes via the electrophilic addition mechanism. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a. In cl 2 molecule, each chlorine atom. Chlorine is in group 7 of the. When. Chlorine Has How Many Bonds.
From chamotgallery.com
How many protons, neutrons and electrons does chlorine have? (2023) Chlorine Has How Many Bonds When two chlorine atoms form a chlorine molecule, they share one pair of electrons. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a. Chlorine can also react with alkenes via the electrophilic addition mechanism. Why. Chlorine Has How Many Bonds.
From www.numerade.com
SOLVED CIF4 How many single bonds are on the chlorine? How many double Chlorine Has How Many Bonds Diatomic molecules such as hydrogen (\(\ce{h2}\)), chlorine (\(\ce{cl2}\)), fluorine (\(\ce{f2}\)), etc. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a. Chlorine can also react with alkenes via the electrophilic addition mechanism. In cl 2 molecule,. Chlorine Has How Many Bonds.
From philschatz.com
Chemical Bonds · Anatomy and Physiology Chlorine Has How Many Bonds Diatomic molecules such as hydrogen (\(\ce{h2}\)), chlorine (\(\ce{cl2}\)), fluorine (\(\ce{f2}\)), etc. This time two chlorine atoms add to a molecule across the. Why does a chlorine molecule have a covalent bond? An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative. Chlorine Has How Many Bonds.
From womackthille.blogspot.com
Expanded Electron Configuration of Chlorine Womack Thille Chlorine Has How Many Bonds An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a. Why does a chlorine molecule have a covalent bond? When two chlorine atoms form a chlorine molecule, they share one pair of electrons. Chlorine can also. Chlorine Has How Many Bonds.
From klarebergsskolan9b.blogspot.com
NO/Teknik 9B Chlorine Has How Many Bonds Why does a chlorine molecule have a covalent bond? Diatomic molecules such as hydrogen (\(\ce{h2}\)), chlorine (\(\ce{cl2}\)), fluorine (\(\ce{f2}\)), etc. This time two chlorine atoms add to a molecule across the. Chlorine is in group 7 of the. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the. Chlorine Has How Many Bonds.
From www.gauthmath.com
Solved Chlorine has 7 valence electrons. Hydrogen has 1 valence Chlorine Has How Many Bonds An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a. Chlorine can also react with alkenes via the electrophilic addition mechanism. In cl 2 molecule, each chlorine atom. A chlorine atom has 7 electrons in its. Chlorine Has How Many Bonds.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons Chlorine Has How Many Bonds An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a. In cl 2 molecule, each chlorine atom. Diatomic molecules such as hydrogen (\(\ce{h2}\)), chlorine (\(\ce{cl2}\)), fluorine (\(\ce{f2}\)), etc. A chlorine atom has 7 electrons in its. Chlorine Has How Many Bonds.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Chlorine Has How Many Bonds Why does a chlorine molecule have a covalent bond? This time two chlorine atoms add to a molecule across the. A chlorine atom has 7 electrons in its outer shell. Chlorine can also react with alkenes via the electrophilic addition mechanism. Diatomic molecules such as hydrogen (\(\ce{h2}\)), chlorine (\(\ce{cl2}\)), fluorine (\(\ce{f2}\)), etc. In cl 2 molecule, each chlorine atom. An. Chlorine Has How Many Bonds.
From gerahellme.weebly.com
__TOP__ How Many Covalent Bonds Can Chlorine Form Chlorine Has How Many Bonds A chlorine atom has 7 electrons in its outer shell. Why does a chlorine molecule have a covalent bond? This time two chlorine atoms add to a molecule across the. Chlorine is in group 7 of the. When two chlorine atoms form a chlorine molecule, they share one pair of electrons. Chlorine can also react with alkenes via the electrophilic. Chlorine Has How Many Bonds.
From brokeasshome.com
Periodic Table Chlorine Electrons Chlorine Has How Many Bonds In cl 2 molecule, each chlorine atom. Chlorine is in group 7 of the. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a. Why does a chlorine molecule have a covalent bond? This time two. Chlorine Has How Many Bonds.
From www.shalom-education.com
Covalent Bonding GCSE Chemistry Revision Chlorine Has How Many Bonds Chlorine is in group 7 of the. When two chlorine atoms form a chlorine molecule, they share one pair of electrons. Chlorine can also react with alkenes via the electrophilic addition mechanism. In cl 2 molecule, each chlorine atom. This time two chlorine atoms add to a molecule across the. Why does a chlorine molecule have a covalent bond? An. Chlorine Has How Many Bonds.
From newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Chlorine Has How Many Bonds A chlorine atom has 7 electrons in its outer shell. Chlorine can also react with alkenes via the electrophilic addition mechanism. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a. Chlorine is in group 7. Chlorine Has How Many Bonds.
From utedzz.blogspot.com
Periodic Table Chlorine Atomic Number Periodic Table Timeline Chlorine Has How Many Bonds This time two chlorine atoms add to a molecule across the. In cl 2 molecule, each chlorine atom. Why does a chlorine molecule have a covalent bond? When two chlorine atoms form a chlorine molecule, they share one pair of electrons. Diatomic molecules such as hydrogen (\(\ce{h2}\)), chlorine (\(\ce{cl2}\)), fluorine (\(\ce{f2}\)), etc. An atom of sodium (na) donates one of. Chlorine Has How Many Bonds.
From www.slideserve.com
PPT Chemical Bonds The Formation of Compounds From Atoms PowerPoint Chlorine Has How Many Bonds An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a. This time two chlorine atoms add to a molecule across the. In cl 2 molecule, each chlorine atom. A chlorine atom has 7 electrons in its. Chlorine Has How Many Bonds.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image Chlorine Has How Many Bonds Diatomic molecules such as hydrogen (\(\ce{h2}\)), chlorine (\(\ce{cl2}\)), fluorine (\(\ce{f2}\)), etc. Chlorine can also react with alkenes via the electrophilic addition mechanism. A chlorine atom has 7 electrons in its outer shell. When two chlorine atoms form a chlorine molecule, they share one pair of electrons. Why does a chlorine molecule have a covalent bond? Chlorine is in group 7. Chlorine Has How Many Bonds.
From wisc.pb.unizin.org
M8Q3 Resonance Structures and Formal Charge Chem 103/104 Resource Book Chlorine Has How Many Bonds An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a. A chlorine atom has 7 electrons in its outer shell. In cl 2 molecule, each chlorine atom. When two chlorine atoms form a chlorine molecule, they. Chlorine Has How Many Bonds.
From fineartamerica.com
Bond Formation In Chlorine Molecule Photograph by Chlorine Has How Many Bonds Why does a chlorine molecule have a covalent bond? This time two chlorine atoms add to a molecule across the. In cl 2 molecule, each chlorine atom. Chlorine is in group 7 of the. Diatomic molecules such as hydrogen (\(\ce{h2}\)), chlorine (\(\ce{cl2}\)), fluorine (\(\ce{f2}\)), etc. When two chlorine atoms form a chlorine molecule, they share one pair of electrons. Chlorine. Chlorine Has How Many Bonds.
From www.youtube.com
ClF5 Lewis Structure How to Draw the Lewis Structure for ClF5 Chlorine Has How Many Bonds When two chlorine atoms form a chlorine molecule, they share one pair of electrons. Chlorine can also react with alkenes via the electrophilic addition mechanism. Why does a chlorine molecule have a covalent bond? In cl 2 molecule, each chlorine atom. Chlorine is in group 7 of the. An atom of sodium (na) donates one of its electrons to an. Chlorine Has How Many Bonds.
From www.chemistrystudent.com
Covalent Bonding (ALevel) ChemistryStudent Chlorine Has How Many Bonds A chlorine atom has 7 electrons in its outer shell. Why does a chlorine molecule have a covalent bond? Chlorine can also react with alkenes via the electrophilic addition mechanism. Diatomic molecules such as hydrogen (\(\ce{h2}\)), chlorine (\(\ce{cl2}\)), fluorine (\(\ce{f2}\)), etc. In cl 2 molecule, each chlorine atom. When two chlorine atoms form a chlorine molecule, they share one pair. Chlorine Has How Many Bonds.
From courses.lumenlearning.com
Chemical Bonds BIO103 Human Biology Chlorine Has How Many Bonds This time two chlorine atoms add to a molecule across the. Chlorine is in group 7 of the. When two chlorine atoms form a chlorine molecule, they share one pair of electrons. Why does a chlorine molecule have a covalent bond? Chlorine can also react with alkenes via the electrophilic addition mechanism. An atom of sodium (na) donates one of. Chlorine Has How Many Bonds.
From valenceelectrons.com
How Many Valence Electrons Does Chlorine (Cl) Have? Chlorine Has How Many Bonds Chlorine is in group 7 of the. When two chlorine atoms form a chlorine molecule, they share one pair of electrons. Diatomic molecules such as hydrogen (\(\ce{h2}\)), chlorine (\(\ce{cl2}\)), fluorine (\(\ce{f2}\)), etc. A chlorine atom has 7 electrons in its outer shell. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a. Chlorine Has How Many Bonds.
From www.youtube.com
How to Draw the Lewis Structure for ClO2 (Chlorine dioxide) YouTube Chlorine Has How Many Bonds Diatomic molecules such as hydrogen (\(\ce{h2}\)), chlorine (\(\ce{cl2}\)), fluorine (\(\ce{f2}\)), etc. Chlorine is in group 7 of the. In cl 2 molecule, each chlorine atom. A chlorine atom has 7 electrons in its outer shell. Why does a chlorine molecule have a covalent bond? An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl). Chlorine Has How Many Bonds.
From chemsimplified.com
How to Predict number of bonds each element forms ChemSimplified Chlorine Has How Many Bonds Chlorine can also react with alkenes via the electrophilic addition mechanism. Why does a chlorine molecule have a covalent bond? An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a. Chlorine is in group 7 of. Chlorine Has How Many Bonds.
From valenceelectrons.com
How to Find the Valence Electrons for ClF3 (Chlorine Trifluoride)? Chlorine Has How Many Bonds Diatomic molecules such as hydrogen (\(\ce{h2}\)), chlorine (\(\ce{cl2}\)), fluorine (\(\ce{f2}\)), etc. Why does a chlorine molecule have a covalent bond? An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a. Chlorine can also react with alkenes. Chlorine Has How Many Bonds.
From www.youtube.com
Covalent Bond of Hydrogen , Chlorine and Hydro Chloride Molecule Chlorine Has How Many Bonds Chlorine can also react with alkenes via the electrophilic addition mechanism. When two chlorine atoms form a chlorine molecule, they share one pair of electrons. Chlorine is in group 7 of the. Why does a chlorine molecule have a covalent bond? Diatomic molecules such as hydrogen (\(\ce{h2}\)), chlorine (\(\ce{cl2}\)), fluorine (\(\ce{f2}\)), etc. In cl 2 molecule, each chlorine atom. A. Chlorine Has How Many Bonds.
From www.teachoo.com
Isotopes and Isobars Definition, Uses and Difference Teachoo Chlorine Has How Many Bonds Chlorine can also react with alkenes via the electrophilic addition mechanism. Diatomic molecules such as hydrogen (\(\ce{h2}\)), chlorine (\(\ce{cl2}\)), fluorine (\(\ce{f2}\)), etc. A chlorine atom has 7 electrons in its outer shell. In cl 2 molecule, each chlorine atom. This time two chlorine atoms add to a molecule across the. Why does a chlorine molecule have a covalent bond? Chlorine. Chlorine Has How Many Bonds.
From chemistry98.blogspot.com
Chem Easy Formation of covalent bond in chlorine molecule Chlorine Has How Many Bonds An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a. When two chlorine atoms form a chlorine molecule, they share one pair of electrons. Why does a chlorine molecule have a covalent bond? This time two. Chlorine Has How Many Bonds.
From byjus.com
The total number of electrons shared between the atoms of a hydrogen Chlorine Has How Many Bonds An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a. Chlorine is in group 7 of the. In cl 2 molecule, each chlorine atom. A chlorine atom has 7 electrons in its outer shell. Why does. Chlorine Has How Many Bonds.
From socratic.org
Chlorine combined with two negative atom or 1 positive and other Chlorine Has How Many Bonds In cl 2 molecule, each chlorine atom. Diatomic molecules such as hydrogen (\(\ce{h2}\)), chlorine (\(\ce{cl2}\)), fluorine (\(\ce{f2}\)), etc. Why does a chlorine molecule have a covalent bond? When two chlorine atoms form a chlorine molecule, they share one pair of electrons. Chlorine is in group 7 of the. An atom of sodium (na) donates one of its electrons to an. Chlorine Has How Many Bonds.
From thesciencecore.blogspot.com
covalent bond definition, properties and examples The Science Core Chlorine Has How Many Bonds Chlorine is in group 7 of the. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a. Diatomic molecules such as hydrogen (\(\ce{h2}\)), chlorine (\(\ce{cl2}\)), fluorine (\(\ce{f2}\)), etc. When two chlorine atoms form a chlorine molecule,. Chlorine Has How Many Bonds.
From www.youtube.com
Atomic Structure (Bohr Model) for Chlorine (Cl) YouTube Chlorine Has How Many Bonds An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a. Why does a chlorine molecule have a covalent bond? In cl 2 molecule, each chlorine atom. When two chlorine atoms form a chlorine molecule, they share. Chlorine Has How Many Bonds.
From www.chemistrylearner.com
Chemical Bonds Definition, Types, and Examples Chlorine Has How Many Bonds A chlorine atom has 7 electrons in its outer shell. Chlorine is in group 7 of the. In cl 2 molecule, each chlorine atom. This time two chlorine atoms add to a molecule across the. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion. Chlorine Has How Many Bonds.