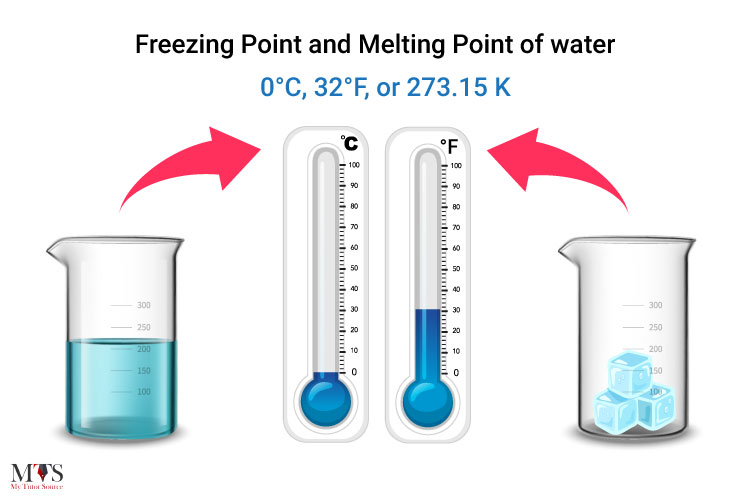
What Temperature Does Liquid Freeze . The freezing point of a liquid or the melting point of. When water freezes, the molecules slow and settle into place, lining up in regular formations you see as crystals. As with the melting point, increased pressure usually raises the freezing. For pure water, the temperature must drop to 32 degrees. The temperature of a freezing liquid remains constant, even when more heat is removed. Freezing point, temperature at which a liquid becomes a solid. The freezing point of water describes the point at which water transitions from a liquid to a solid (ice), while the melting point is the temperature at which water turns from a.
from ksa.mytutorsource.com
As with the melting point, increased pressure usually raises the freezing. For pure water, the temperature must drop to 32 degrees. The freezing point of water describes the point at which water transitions from a liquid to a solid (ice), while the melting point is the temperature at which water turns from a. The freezing point of a liquid or the melting point of. When water freezes, the molecules slow and settle into place, lining up in regular formations you see as crystals. Freezing point, temperature at which a liquid becomes a solid. The temperature of a freezing liquid remains constant, even when more heat is removed.
Water Freezing Point Definition, Factors Affecting It & Supercooled
What Temperature Does Liquid Freeze The freezing point of water describes the point at which water transitions from a liquid to a solid (ice), while the melting point is the temperature at which water turns from a. For pure water, the temperature must drop to 32 degrees. The freezing point of water describes the point at which water transitions from a liquid to a solid (ice), while the melting point is the temperature at which water turns from a. When water freezes, the molecules slow and settle into place, lining up in regular formations you see as crystals. The freezing point of a liquid or the melting point of. As with the melting point, increased pressure usually raises the freezing. Freezing point, temperature at which a liquid becomes a solid. The temperature of a freezing liquid remains constant, even when more heat is removed.
From smartclass4kids.com
Changing States of Matter Solid, Liquid,Gas, Phase Change What Temperature Does Liquid Freeze As with the melting point, increased pressure usually raises the freezing. Freezing point, temperature at which a liquid becomes a solid. The freezing point of a liquid or the melting point of. For pure water, the temperature must drop to 32 degrees. The temperature of a freezing liquid remains constant, even when more heat is removed. When water freezes, the. What Temperature Does Liquid Freeze.
From www.thoughtco.com
What Is the Freezing Point of Water? What Temperature Does Liquid Freeze The temperature of a freezing liquid remains constant, even when more heat is removed. For pure water, the temperature must drop to 32 degrees. The freezing point of water describes the point at which water transitions from a liquid to a solid (ice), while the melting point is the temperature at which water turns from a. The freezing point of. What Temperature Does Liquid Freeze.
From www.pinterest.com
Freezing Point Easy Science Chemistry education, Science flashcards What Temperature Does Liquid Freeze As with the melting point, increased pressure usually raises the freezing. The freezing point of a liquid or the melting point of. The freezing point of water describes the point at which water transitions from a liquid to a solid (ice), while the melting point is the temperature at which water turns from a. For pure water, the temperature must. What Temperature Does Liquid Freeze.
From www.worldatlas.com
What is the Freezing Point in Celsius? WorldAtlas What Temperature Does Liquid Freeze The freezing point of water describes the point at which water transitions from a liquid to a solid (ice), while the melting point is the temperature at which water turns from a. The temperature of a freezing liquid remains constant, even when more heat is removed. The freezing point of a liquid or the melting point of. As with the. What Temperature Does Liquid Freeze.
From www.dreamstime.com
Boiling and Evaporation, Freezing and Melting Points of Water Stock What Temperature Does Liquid Freeze When water freezes, the molecules slow and settle into place, lining up in regular formations you see as crystals. The freezing point of water describes the point at which water transitions from a liquid to a solid (ice), while the melting point is the temperature at which water turns from a. The temperature of a freezing liquid remains constant, even. What Temperature Does Liquid Freeze.
From tipseri.com
At what temperature does sea water freeze Celsius? Tipseri What Temperature Does Liquid Freeze Freezing point, temperature at which a liquid becomes a solid. When water freezes, the molecules slow and settle into place, lining up in regular formations you see as crystals. The freezing point of water describes the point at which water transitions from a liquid to a solid (ice), while the melting point is the temperature at which water turns from. What Temperature Does Liquid Freeze.
From mungfali.com
At What Temperature Does Water Freeze What Temperature Does Liquid Freeze As with the melting point, increased pressure usually raises the freezing. For pure water, the temperature must drop to 32 degrees. The temperature of a freezing liquid remains constant, even when more heat is removed. When water freezes, the molecules slow and settle into place, lining up in regular formations you see as crystals. The freezing point of a liquid. What Temperature Does Liquid Freeze.
From gabriela-classwork.blogspot.com
Science Class septiembre 2010 What Temperature Does Liquid Freeze The temperature of a freezing liquid remains constant, even when more heat is removed. The freezing point of a liquid or the melting point of. For pure water, the temperature must drop to 32 degrees. When water freezes, the molecules slow and settle into place, lining up in regular formations you see as crystals. The freezing point of water describes. What Temperature Does Liquid Freeze.
From icytales.com
Water Freezing Temperature Surprising Facts Revealed What Temperature Does Liquid Freeze Freezing point, temperature at which a liquid becomes a solid. For pure water, the temperature must drop to 32 degrees. The freezing point of a liquid or the melting point of. When water freezes, the molecules slow and settle into place, lining up in regular formations you see as crystals. The temperature of a freezing liquid remains constant, even when. What Temperature Does Liquid Freeze.
From www.rmets.org
The highs, lows and feels of temperature Royal Meteorological Society What Temperature Does Liquid Freeze The freezing point of water describes the point at which water transitions from a liquid to a solid (ice), while the melting point is the temperature at which water turns from a. The freezing point of a liquid or the melting point of. Freezing point, temperature at which a liquid becomes a solid. As with the melting point, increased pressure. What Temperature Does Liquid Freeze.
From ksa.mytutorsource.com
Water Freezing Point Definition, Factors Affecting It & Supercooled What Temperature Does Liquid Freeze As with the melting point, increased pressure usually raises the freezing. The freezing point of water describes the point at which water transitions from a liquid to a solid (ice), while the melting point is the temperature at which water turns from a. When water freezes, the molecules slow and settle into place, lining up in regular formations you see. What Temperature Does Liquid Freeze.
From www.launchknowledge.com
What Temp Does Salt Water Freeze What Temperature Does Liquid Freeze The freezing point of a liquid or the melting point of. As with the melting point, increased pressure usually raises the freezing. For pure water, the temperature must drop to 32 degrees. Freezing point, temperature at which a liquid becomes a solid. The freezing point of water describes the point at which water transitions from a liquid to a solid. What Temperature Does Liquid Freeze.
From www.youtube.com
At what temperature does water freeze at Celsius? YouTube What Temperature Does Liquid Freeze The temperature of a freezing liquid remains constant, even when more heat is removed. The freezing point of water describes the point at which water transitions from a liquid to a solid (ice), while the melting point is the temperature at which water turns from a. When water freezes, the molecules slow and settle into place, lining up in regular. What Temperature Does Liquid Freeze.
From www.slideserve.com
PPT Chapter 6 Section 2 PowerPoint Presentation, free download ID What Temperature Does Liquid Freeze As with the melting point, increased pressure usually raises the freezing. Freezing point, temperature at which a liquid becomes a solid. When water freezes, the molecules slow and settle into place, lining up in regular formations you see as crystals. The freezing point of a liquid or the melting point of. For pure water, the temperature must drop to 32. What Temperature Does Liquid Freeze.
From brainly.com
Water freezes at a temperature of _____. A. 0 °C B. 0 °F C. 100 °C D What Temperature Does Liquid Freeze The temperature of a freezing liquid remains constant, even when more heat is removed. For pure water, the temperature must drop to 32 degrees. The freezing point of water describes the point at which water transitions from a liquid to a solid (ice), while the melting point is the temperature at which water turns from a. As with the melting. What Temperature Does Liquid Freeze.
From fyownpivb.blob.core.windows.net
What Temperature Does Running Water Freeze In Pipes at Aline Nelson blog What Temperature Does Liquid Freeze When water freezes, the molecules slow and settle into place, lining up in regular formations you see as crystals. The freezing point of water describes the point at which water transitions from a liquid to a solid (ice), while the melting point is the temperature at which water turns from a. Freezing point, temperature at which a liquid becomes a. What Temperature Does Liquid Freeze.
From www.cuemath.com
Celsius to Fahrenheit Conversion Formula Examples What Temperature Does Liquid Freeze Freezing point, temperature at which a liquid becomes a solid. As with the melting point, increased pressure usually raises the freezing. When water freezes, the molecules slow and settle into place, lining up in regular formations you see as crystals. The freezing point of water describes the point at which water transitions from a liquid to a solid (ice), while. What Temperature Does Liquid Freeze.
From hvacseer.com
At What Temperature Do Pipes Freeze? What Temperature Does Liquid Freeze As with the melting point, increased pressure usually raises the freezing. The freezing point of a liquid or the melting point of. Freezing point, temperature at which a liquid becomes a solid. The temperature of a freezing liquid remains constant, even when more heat is removed. For pure water, the temperature must drop to 32 degrees. When water freezes, the. What Temperature Does Liquid Freeze.
From vectormine.com
Absolute zero as lowest temperature limit for water freezing outline What Temperature Does Liquid Freeze When water freezes, the molecules slow and settle into place, lining up in regular formations you see as crystals. The freezing point of a liquid or the melting point of. The temperature of a freezing liquid remains constant, even when more heat is removed. Freezing point, temperature at which a liquid becomes a solid. As with the melting point, increased. What Temperature Does Liquid Freeze.
From www.pinterest.com
Water Expansion When Freezing Science Facts Water molecule What Temperature Does Liquid Freeze As with the melting point, increased pressure usually raises the freezing. When water freezes, the molecules slow and settle into place, lining up in regular formations you see as crystals. The temperature of a freezing liquid remains constant, even when more heat is removed. The freezing point of a liquid or the melting point of. Freezing point, temperature at which. What Temperature Does Liquid Freeze.
From www.reddit.com
What happens to water that is put into freezing temperature but unable What Temperature Does Liquid Freeze When water freezes, the molecules slow and settle into place, lining up in regular formations you see as crystals. As with the melting point, increased pressure usually raises the freezing. For pure water, the temperature must drop to 32 degrees. The temperature of a freezing liquid remains constant, even when more heat is removed. The freezing point of a liquid. What Temperature Does Liquid Freeze.
From www.worksheetsplanet.com
What is Freezing Definition of Freezing What Temperature Does Liquid Freeze The temperature of a freezing liquid remains constant, even when more heat is removed. For pure water, the temperature must drop to 32 degrees. When water freezes, the molecules slow and settle into place, lining up in regular formations you see as crystals. The freezing point of water describes the point at which water transitions from a liquid to a. What Temperature Does Liquid Freeze.
From www.metoffice.gov.uk
Sea ice an overview Met Office What Temperature Does Liquid Freeze The freezing point of a liquid or the melting point of. For pure water, the temperature must drop to 32 degrees. Freezing point, temperature at which a liquid becomes a solid. When water freezes, the molecules slow and settle into place, lining up in regular formations you see as crystals. As with the melting point, increased pressure usually raises the. What Temperature Does Liquid Freeze.
From www.gettyimages.ie
Freezing And Boiling Points In Celsius And Fahrenheit HighRes Vector What Temperature Does Liquid Freeze The freezing point of a liquid or the melting point of. As with the melting point, increased pressure usually raises the freezing. Freezing point, temperature at which a liquid becomes a solid. The temperature of a freezing liquid remains constant, even when more heat is removed. For pure water, the temperature must drop to 32 degrees. The freezing point of. What Temperature Does Liquid Freeze.
From www.slideserve.com
PPT Chapter 6 Section 2 PowerPoint Presentation, free download ID What Temperature Does Liquid Freeze As with the melting point, increased pressure usually raises the freezing. Freezing point, temperature at which a liquid becomes a solid. The temperature of a freezing liquid remains constant, even when more heat is removed. For pure water, the temperature must drop to 32 degrees. The freezing point of water describes the point at which water transitions from a liquid. What Temperature Does Liquid Freeze.
From jacksofscience.com
What Temp Does Salt Water Freeze? Jacks Of Science What Temperature Does Liquid Freeze The freezing point of water describes the point at which water transitions from a liquid to a solid (ice), while the melting point is the temperature at which water turns from a. For pure water, the temperature must drop to 32 degrees. Freezing point, temperature at which a liquid becomes a solid. When water freezes, the molecules slow and settle. What Temperature Does Liquid Freeze.
From www.slideserve.com
PPT Freezing/Melting and Boiling Points PowerPoint Presentation, free What Temperature Does Liquid Freeze When water freezes, the molecules slow and settle into place, lining up in regular formations you see as crystals. The freezing point of a liquid or the melting point of. For pure water, the temperature must drop to 32 degrees. As with the melting point, increased pressure usually raises the freezing. The temperature of a freezing liquid remains constant, even. What Temperature Does Liquid Freeze.
From www.wtamu.edu
Can water stay liquid below zero degrees Celsius? Science Questions What Temperature Does Liquid Freeze The freezing point of water describes the point at which water transitions from a liquid to a solid (ice), while the melting point is the temperature at which water turns from a. The temperature of a freezing liquid remains constant, even when more heat is removed. As with the melting point, increased pressure usually raises the freezing. Freezing point, temperature. What Temperature Does Liquid Freeze.
From ksa.mytutorsource.com
Water Freezing Point Definition, Factors Affecting It & Supercooled What Temperature Does Liquid Freeze When water freezes, the molecules slow and settle into place, lining up in regular formations you see as crystals. The freezing point of water describes the point at which water transitions from a liquid to a solid (ice), while the melting point is the temperature at which water turns from a. For pure water, the temperature must drop to 32. What Temperature Does Liquid Freeze.
From jacksofscience.com
What Temp Does Salt Water Freeze? Jacks Of Science What Temperature Does Liquid Freeze When water freezes, the molecules slow and settle into place, lining up in regular formations you see as crystals. Freezing point, temperature at which a liquid becomes a solid. As with the melting point, increased pressure usually raises the freezing. The freezing point of a liquid or the melting point of. For pure water, the temperature must drop to 32. What Temperature Does Liquid Freeze.
From whatsinsight.org
What Temp Does Water Freeze? Science What's Insight What Temperature Does Liquid Freeze As with the melting point, increased pressure usually raises the freezing. The freezing point of water describes the point at which water transitions from a liquid to a solid (ice), while the melting point is the temperature at which water turns from a. The temperature of a freezing liquid remains constant, even when more heat is removed. The freezing point. What Temperature Does Liquid Freeze.
From blograng.com
Can water freeze in 20 minutes? What Temperature Does Liquid Freeze When water freezes, the molecules slow and settle into place, lining up in regular formations you see as crystals. The freezing point of water describes the point at which water transitions from a liquid to a solid (ice), while the melting point is the temperature at which water turns from a. As with the melting point, increased pressure usually raises. What Temperature Does Liquid Freeze.
From www.researchgate.net
Typical freezing curve of water Download Scientific Diagram What Temperature Does Liquid Freeze The temperature of a freezing liquid remains constant, even when more heat is removed. The freezing point of a liquid or the melting point of. Freezing point, temperature at which a liquid becomes a solid. The freezing point of water describes the point at which water transitions from a liquid to a solid (ice), while the melting point is the. What Temperature Does Liquid Freeze.
From www.wisegeek.com
What Are the Properties of Liquids? (with picture) What Temperature Does Liquid Freeze For pure water, the temperature must drop to 32 degrees. The temperature of a freezing liquid remains constant, even when more heat is removed. The freezing point of water describes the point at which water transitions from a liquid to a solid (ice), while the melting point is the temperature at which water turns from a. The freezing point of. What Temperature Does Liquid Freeze.
From sciencenotes.org
What Is the Freezing Point of Water? Fahrenheit, Celsius, and Kelvin What Temperature Does Liquid Freeze The freezing point of water describes the point at which water transitions from a liquid to a solid (ice), while the melting point is the temperature at which water turns from a. The freezing point of a liquid or the melting point of. For pure water, the temperature must drop to 32 degrees. As with the melting point, increased pressure. What Temperature Does Liquid Freeze.