Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation . The balanced equation will be calculated along with the. A solution of lead(ii) nitrate is. Enter an equation of an ionic chemical equation and press the balance button. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly involved in the chemical. All salts of nitrates, chlorates and acetates are soluble. The balanced molecular equation for the reaction between copper(ii) sulfate (cuso a 4) and lead(ii) nitrat. Let's first start with a complete chemical equation and see how the net ionic equation is derived. For example, take the reaction of lead(ii) nitrate. If precipitation is expected, write a. All salts of halides are soluble except those of silver(i), copper(i), lead(ii), and.
from cristianmeowterry.blogspot.com
The balanced equation will be calculated along with the. A solution of lead(ii) nitrate is. Let's first start with a complete chemical equation and see how the net ionic equation is derived. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. The balanced molecular equation for the reaction between copper(ii) sulfate (cuso a 4) and lead(ii) nitrat. All salts of halides are soluble except those of silver(i), copper(i), lead(ii), and. The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly involved in the chemical. For example, take the reaction of lead(ii) nitrate. If precipitation is expected, write a. All salts of nitrates, chlorates and acetates are soluble.
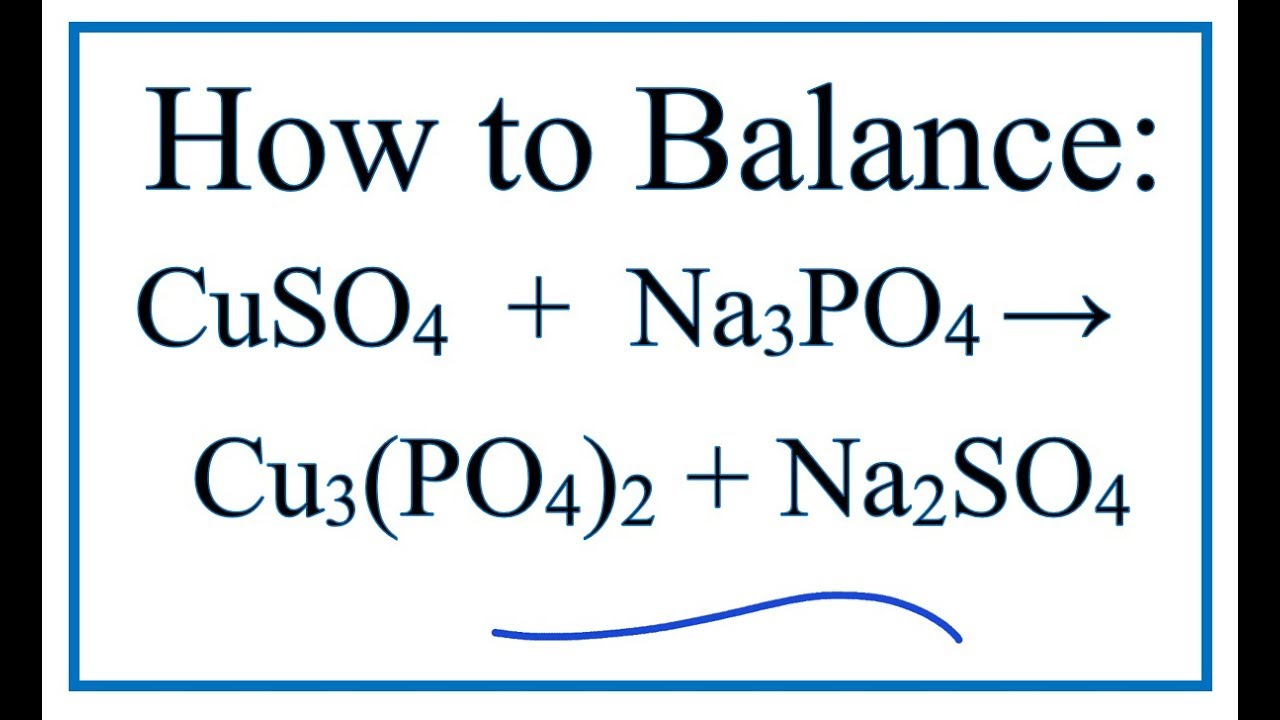
Molecular Equation for Copper Ii Sulfate and Sodium Phosphate
Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation A solution of lead(ii) nitrate is. For example, take the reaction of lead(ii) nitrate. All salts of nitrates, chlorates and acetates are soluble. If precipitation is expected, write a. A solution of lead(ii) nitrate is. All salts of halides are soluble except those of silver(i), copper(i), lead(ii), and. The balanced molecular equation for the reaction between copper(ii) sulfate (cuso a 4) and lead(ii) nitrat. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly involved in the chemical. Enter an equation of an ionic chemical equation and press the balance button. Let's first start with a complete chemical equation and see how the net ionic equation is derived. The balanced equation will be calculated along with the.
From www.chegg.com
Solved When aqueous solutions of copper(II) sulfate and Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation Let's first start with a complete chemical equation and see how the net ionic equation is derived. The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly involved in the chemical. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. The balanced equation will be calculated along. Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation.
From www.youtube.com
How to Write the Formula for Copper (II) sulfate YouTube Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation The balanced molecular equation for the reaction between copper(ii) sulfate (cuso a 4) and lead(ii) nitrat. The balanced equation will be calculated along with the. If precipitation is expected, write a. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. The net ionic equation is the chemical equation that shows only those elements, compounds, and ions. Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation.
From sciencenotes.org
Net Ionic Equation and Complete Ionic Equation Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation The balanced molecular equation for the reaction between copper(ii) sulfate (cuso a 4) and lead(ii) nitrat. All salts of halides are soluble except those of silver(i), copper(i), lead(ii), and. A solution of lead(ii) nitrate is. Enter an equation of an ionic chemical equation and press the balance button. The net ionic equation is the chemical equation that shows only those. Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation.
From www.slideserve.com
PPT Precipitation Reactions (Double Replacement Reactions) PowerPoint Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly involved in the chemical. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. If precipitation is expected, write a. Let's first start with a complete chemical equation and see how the net ionic equation is derived. Enter. Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation.
From www.numerade.com
SOLVED Give the complete ionic equation for the reaction (if any) that Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation The balanced equation will be calculated along with the. All salts of halides are soluble except those of silver(i), copper(i), lead(ii), and. Let's first start with a complete chemical equation and see how the net ionic equation is derived. If precipitation is expected, write a. All salts of nitrates, chlorates and acetates are soluble. The balanced molecular equation for the. Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation.
From informacionpublica.svet.gob.gt
How To Write The Net Ionic Equation For CuSO4 Na2S Na2SO4 Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation A solution of lead(ii) nitrate is. All salts of halides are soluble except those of silver(i), copper(i), lead(ii), and. If precipitation is expected, write a. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. All salts of nitrates, chlorates and acetates are soluble. For example, take the reaction of lead(ii) nitrate. The balanced equation will be. Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation.
From www.numerade.com
SOLVED Write the balanced net ionic equation for the reaction of Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation All salts of nitrates, chlorates and acetates are soluble. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly involved in the chemical. For example, take the reaction of lead(ii) nitrate. The balanced molecular equation for the reaction. Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation.
From www.wikihow.com
How to Write a Net Ionic Equation 10 Steps (with Pictures) Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation The balanced molecular equation for the reaction between copper(ii) sulfate (cuso a 4) and lead(ii) nitrat. All salts of halides are soluble except those of silver(i), copper(i), lead(ii), and. The balanced equation will be calculated along with the. For example, take the reaction of lead(ii) nitrate. Let's first start with a complete chemical equation and see how the net ionic. Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation.
From baileeghopguzman.blogspot.com
Copper Ii Sulfate and Mercury I Nitrate Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation All salts of nitrates, chlorates and acetates are soluble. The balanced equation will be calculated along with the. Let's first start with a complete chemical equation and see how the net ionic equation is derived. The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly involved in the chemical. Enter an. Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation.
From wayneferscarlson.blogspot.com
Is Lead Nitrate Ionic or Covalent Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation All salts of halides are soluble except those of silver(i), copper(i), lead(ii), and. For example, take the reaction of lead(ii) nitrate. The balanced equation will be calculated along with the. The balanced molecular equation for the reaction between copper(ii) sulfate (cuso a 4) and lead(ii) nitrat. Enter an equation of an ionic chemical equation and press the balance button. If. Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation.
From www.onlinemathlearning.com
Writing Ionic Equation (video lessons, examples and solutions) Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation A solution of lead(ii) nitrate is. The balanced molecular equation for the reaction between copper(ii) sulfate (cuso a 4) and lead(ii) nitrat. The balanced equation will be calculated along with the. Let's first start with a complete chemical equation and see how the net ionic equation is derived. Predict the result of mixing reasonably concentrated solutions of the following ionic. Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation.
From www.chegg.com
Solved What is the net ionic equation for Copper (ii) Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation All salts of halides are soluble except those of silver(i), copper(i), lead(ii), and. For example, take the reaction of lead(ii) nitrate. Enter an equation of an ionic chemical equation and press the balance button. All salts of nitrates, chlorates and acetates are soluble. The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that. Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation.
From www.slideserve.com
PPT Reactions Involving Ions Molecular vs. Ionic Equations Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation All salts of halides are soluble except those of silver(i), copper(i), lead(ii), and. Enter an equation of an ionic chemical equation and press the balance button. All salts of nitrates, chlorates and acetates are soluble. The balanced molecular equation for the reaction between copper(ii) sulfate (cuso a 4) and lead(ii) nitrat. Let's first start with a complete chemical equation and. Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation.
From www.numerade.com
SOLVED Write the balanced net ionic equation for the reaction of Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation Enter an equation of an ionic chemical equation and press the balance button. If precipitation is expected, write a. The balanced equation will be calculated along with the. The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly involved in the chemical. Predict the result of mixing reasonably concentrated solutions of. Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation.
From baileeghopguzman.blogspot.com
Copper Ii Sulfate and Mercury I Nitrate Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation If precipitation is expected, write a. All salts of nitrates, chlorates and acetates are soluble. The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly involved in the chemical. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. The balanced molecular equation for the reaction between copper(ii). Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation.
From www.youtube.com
Precipitation Reactions & Net Ionic Equations Chemistry YouTube Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation Enter an equation of an ionic chemical equation and press the balance button. Let's first start with a complete chemical equation and see how the net ionic equation is derived. The balanced molecular equation for the reaction between copper(ii) sulfate (cuso a 4) and lead(ii) nitrat. All salts of halides are soluble except those of silver(i), copper(i), lead(ii), and. If. Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation.
From www.chegg.com
Solved What is the net ionic equation for the reaction of Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation A solution of lead(ii) nitrate is. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. For example, take the reaction of lead(ii) nitrate. All salts of nitrates, chlorates and acetates are soluble. The balanced molecular equation for the reaction between copper(ii) sulfate (cuso a 4) and lead(ii) nitrat. The net ionic equation is the chemical equation. Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation.
From www.chegg.com
Solved What is the net ionic equation for copper(II) Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly involved in the chemical. A solution of lead(ii) nitrate is. The balanced molecular equation for the reaction between copper(ii) sulfate (cuso a 4) and lead(ii) nitrat. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. The balanced. Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation.
From www.numerade.com
SOLVEDDoes a reaction occur when aqueous solutions of sodium sulfate Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation A solution of lead(ii) nitrate is. The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly involved in the chemical. All salts of nitrates, chlorates and acetates are soluble. Let's first start with a complete chemical equation and see how the net ionic equation is derived. All salts of halides are. Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation.
From www.coursehero.com
[Solved] copper(II)sulfate + Barium chloride molecular equation and Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly involved in the chemical. All salts of nitrates, chlorates and acetates are soluble. Enter an equation of an ionic chemical equation and press the balance button. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. A solution. Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation.
From shotprofessional22.gitlab.io
Beautiful Silver Nitrate And Copper Ionic Equation Edexcel Igcse Maths Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation For example, take the reaction of lead(ii) nitrate. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. The balanced equation will be calculated along with the. All salts of halides are soluble except those of silver(i), copper(i), lead(ii), and. If precipitation is expected, write a. The balanced molecular equation for the reaction between copper(ii) sulfate (cuso. Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation.
From solvedlib.com
Write net ionic equations for the reaction, if any, t… SolvedLib Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation Let's first start with a complete chemical equation and see how the net ionic equation is derived. All salts of halides are soluble except those of silver(i), copper(i), lead(ii), and. The balanced equation will be calculated along with the. For example, take the reaction of lead(ii) nitrate. All salts of nitrates, chlorates and acetates are soluble. The net ionic equation. Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation.
From oneclass.com
OneClass Write the balanced net ionic equation for the reactions that Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation If precipitation is expected, write a. A solution of lead(ii) nitrate is. The balanced equation will be calculated along with the. Enter an equation of an ionic chemical equation and press the balance button. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. For example, take the reaction of lead(ii) nitrate. All salts of nitrates, chlorates. Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation.
From www.youtube.com
How to Write the Net Ionic Equation for CuSO4 + Na3PO4 =Cu3(PO4)2 Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation The balanced equation will be calculated along with the. All salts of halides are soluble except those of silver(i), copper(i), lead(ii), and. For example, take the reaction of lead(ii) nitrate. Let's first start with a complete chemical equation and see how the net ionic equation is derived. The net ionic equation is the chemical equation that shows only those elements,. Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation.
From www.slideserve.com
PPT Reactions in Aqueous Solution PowerPoint Presentation, free Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation All salts of nitrates, chlorates and acetates are soluble. Enter an equation of an ionic chemical equation and press the balance button. A solution of lead(ii) nitrate is. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. The balanced molecular equation for the reaction between copper(ii) sulfate (cuso a 4) and lead(ii) nitrat. Let's first start. Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation.
From shotprofessional22.gitlab.io
Beautiful Silver Nitrate And Copper Ionic Equation Edexcel Igcse Maths Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation For example, take the reaction of lead(ii) nitrate. The balanced equation will be calculated along with the. Let's first start with a complete chemical equation and see how the net ionic equation is derived. If precipitation is expected, write a. All salts of halides are soluble except those of silver(i), copper(i), lead(ii), and. All salts of nitrates, chlorates and acetates. Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation.
From www.coursehero.com
[Solved] write the molecular, ionic, and net ionic equation Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation If precipitation is expected, write a. The balanced molecular equation for the reaction between copper(ii) sulfate (cuso a 4) and lead(ii) nitrat. A solution of lead(ii) nitrate is. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. All salts of halides are soluble except those of silver(i), copper(i), lead(ii), and. Enter an equation of an ionic. Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation.
From www.numerade.com
⏩SOLVEDCopper(Il) chloride and lead(II) nitrate react in aqueous Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation All salts of nitrates, chlorates and acetates are soluble. Enter an equation of an ionic chemical equation and press the balance button. For example, take the reaction of lead(ii) nitrate. All salts of halides are soluble except those of silver(i), copper(i), lead(ii), and. Let's first start with a complete chemical equation and see how the net ionic equation is derived.. Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation.
From www.youtube.com
How to Write the Net Ionic Equation for Cu(NO3)2 + (NH4)2S = CuS Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation All salts of halides are soluble except those of silver(i), copper(i), lead(ii), and. Let's first start with a complete chemical equation and see how the net ionic equation is derived. The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly involved in the chemical. The balanced molecular equation for the reaction. Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation.
From www.numerade.com
SOLVEDLead ions can be removed from solution by precipitation with Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation All salts of nitrates, chlorates and acetates are soluble. The balanced equation will be calculated along with the. All salts of halides are soluble except those of silver(i), copper(i), lead(ii), and. The balanced molecular equation for the reaction between copper(ii) sulfate (cuso a 4) and lead(ii) nitrat. For example, take the reaction of lead(ii) nitrate. A solution of lead(ii) nitrate. Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation.
From cristianmeowterry.blogspot.com
Molecular Equation for Copper Ii Sulfate and Sodium Phosphate Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation A solution of lead(ii) nitrate is. All salts of nitrates, chlorates and acetates are soluble. All salts of halides are soluble except those of silver(i), copper(i), lead(ii), and. The balanced molecular equation for the reaction between copper(ii) sulfate (cuso a 4) and lead(ii) nitrat. For example, take the reaction of lead(ii) nitrate. The net ionic equation is the chemical equation. Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation.
From www.numerade.com
SOLVEDionic equations Track the electrons lost and gained in the Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation A solution of lead(ii) nitrate is. If precipitation is expected, write a. Let's first start with a complete chemical equation and see how the net ionic equation is derived. Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. For example, take the reaction of lead(ii) nitrate. The net ionic equation is the chemical equation that shows. Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation.
From www.tessshebaylo.com
Net Ionic Equation Examples Tessshebaylo Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation A solution of lead(ii) nitrate is. Let's first start with a complete chemical equation and see how the net ionic equation is derived. All salts of halides are soluble except those of silver(i), copper(i), lead(ii), and. The balanced molecular equation for the reaction between copper(ii) sulfate (cuso a 4) and lead(ii) nitrat. All salts of nitrates, chlorates and acetates are. Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation.
From www.numerade.com
SOLVED Write the net ionic equation for the reaction of copper (II Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation A solution of lead(ii) nitrate is. The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly involved in the chemical. The balanced molecular equation for the reaction between copper(ii) sulfate (cuso a 4) and lead(ii) nitrat. If precipitation is expected, write a. All salts of nitrates, chlorates and acetates are soluble.. Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation.
From slidetodoc.com
SOLUTION S UNIT Introduction Net Ionic Equations What Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation The balanced molecular equation for the reaction between copper(ii) sulfate (cuso a 4) and lead(ii) nitrat. For example, take the reaction of lead(ii) nitrate. The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly involved in the chemical. All salts of halides are soluble except those of silver(i), copper(i), lead(ii), and.. Copper(Ii) Sulfate And Lead Nitrate Net Ionic Equation.