Interface Between Liquids . What is the difference between an interface and a surface? Two basic kinds of level interfaces: This chapter examines the properties of interfaces between a liquid and a fluid (liquid or gas) containing multiple. While the term interfacial tension refers to interfaces between two liquids, the term surface tension refers to the interactions between a liquid and a gaseous phase. An interface is the contact plane between two phases which do not mix. A clear interface between two liquids (left) and an emulsion or rag layer between two liquids (right). This can be seen if droplets of one liquid are dropped into the bulk. A phase is an area in which physical quantities do not. This section addresses the case of interfaces between two fluids onto which a species insoluble in both of them has been. An example is the surface tension. Modern level measurement technology is capable of detecting all kinds of interfaces, including multiple layers.
from www.chegg.com
Two basic kinds of level interfaces: An example is the surface tension. This chapter examines the properties of interfaces between a liquid and a fluid (liquid or gas) containing multiple. A phase is an area in which physical quantities do not. A clear interface between two liquids (left) and an emulsion or rag layer between two liquids (right). While the term interfacial tension refers to interfaces between two liquids, the term surface tension refers to the interactions between a liquid and a gaseous phase. What is the difference between an interface and a surface? An interface is the contact plane between two phases which do not mix. This can be seen if droplets of one liquid are dropped into the bulk. This section addresses the case of interfaces between two fluids onto which a species insoluble in both of them has been.
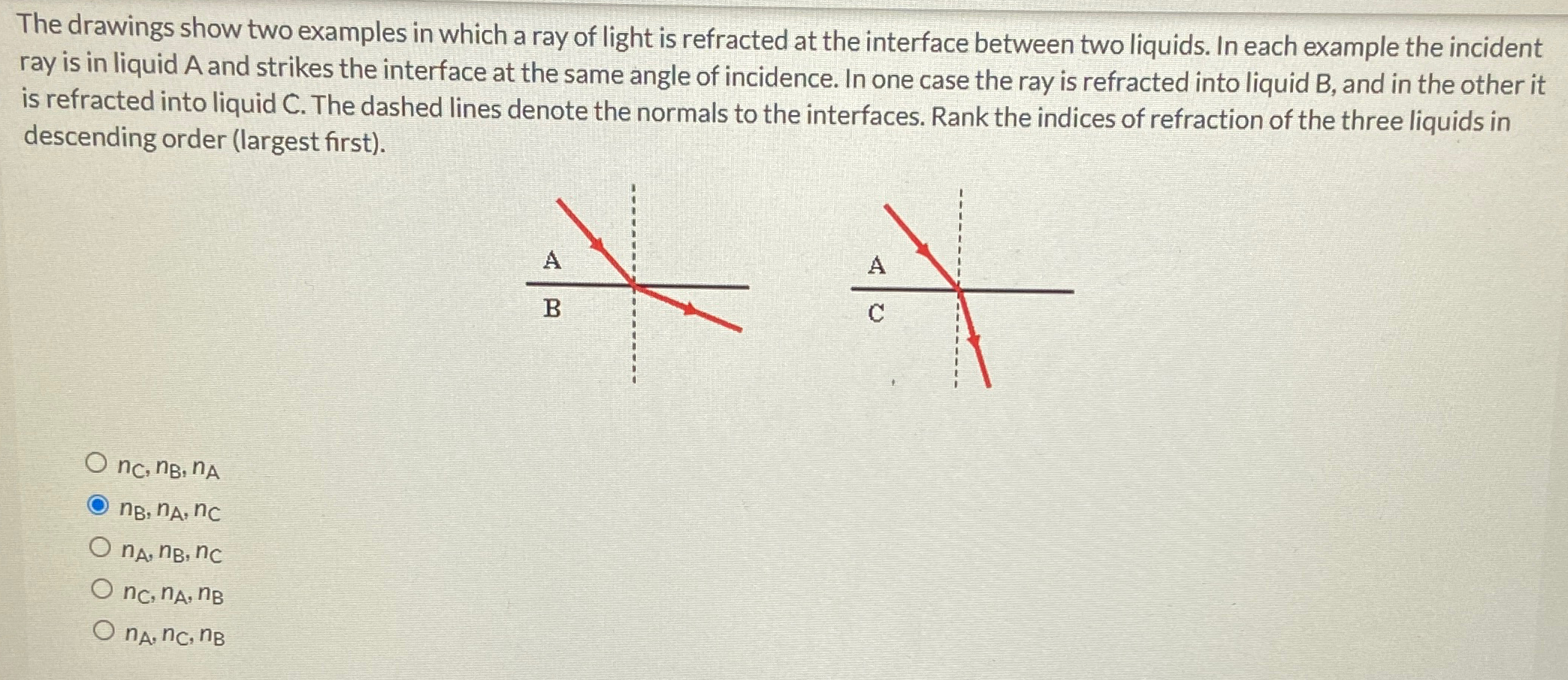
Solved The drawings show two examples in which a ray of
Interface Between Liquids This section addresses the case of interfaces between two fluids onto which a species insoluble in both of them has been. An interface is the contact plane between two phases which do not mix. While the term interfacial tension refers to interfaces between two liquids, the term surface tension refers to the interactions between a liquid and a gaseous phase. This can be seen if droplets of one liquid are dropped into the bulk. What is the difference between an interface and a surface? Two basic kinds of level interfaces: This section addresses the case of interfaces between two fluids onto which a species insoluble in both of them has been. This chapter examines the properties of interfaces between a liquid and a fluid (liquid or gas) containing multiple. A clear interface between two liquids (left) and an emulsion or rag layer between two liquids (right). An example is the surface tension. Modern level measurement technology is capable of detecting all kinds of interfaces, including multiple layers. A phase is an area in which physical quantities do not.
From www.researchgate.net
The reaction products at the airliquid interface based on liquid SIMS Interface Between Liquids This can be seen if droplets of one liquid are dropped into the bulk. Two basic kinds of level interfaces: An example is the surface tension. A clear interface between two liquids (left) and an emulsion or rag layer between two liquids (right). While the term interfacial tension refers to interfaces between two liquids, the term surface tension refers to. Interface Between Liquids.
From publish.illinois.edu
Ionic Liquids at Interfaces Structure and Dynamics EspinosaMarzal Lab Interface Between Liquids Modern level measurement technology is capable of detecting all kinds of interfaces, including multiple layers. A phase is an area in which physical quantities do not. An example is the surface tension. A clear interface between two liquids (left) and an emulsion or rag layer between two liquids (right). This section addresses the case of interfaces between two fluids onto. Interface Between Liquids.
From researchoutreach.org
Facilitated ion transfer through liquidliquid interfaces Research Interface Between Liquids Two basic kinds of level interfaces: This chapter examines the properties of interfaces between a liquid and a fluid (liquid or gas) containing multiple. What is the difference between an interface and a surface? An interface is the contact plane between two phases which do not mix. A phase is an area in which physical quantities do not. While the. Interface Between Liquids.
From pubs.rsc.org
Liquidliquid interfaces a unique and advantageous environment to Interface Between Liquids Two basic kinds of level interfaces: An interface is the contact plane between two phases which do not mix. This section addresses the case of interfaces between two fluids onto which a species insoluble in both of them has been. This can be seen if droplets of one liquid are dropped into the bulk. Modern level measurement technology is capable. Interface Between Liquids.
From www.physik.uni-kiel.de
Crystal growth at liquidliquid interfaces studied with atomic Interface Between Liquids An example is the surface tension. This section addresses the case of interfaces between two fluids onto which a species insoluble in both of them has been. An interface is the contact plane between two phases which do not mix. A clear interface between two liquids (left) and an emulsion or rag layer between two liquids (right). Two basic kinds. Interface Between Liquids.
From www.researchgate.net
Liquidsolid interfaces a, b atomically smooth, c, d atomically rough Interface Between Liquids This chapter examines the properties of interfaces between a liquid and a fluid (liquid or gas) containing multiple. This can be seen if droplets of one liquid are dropped into the bulk. Two basic kinds of level interfaces: An example is the surface tension. A clear interface between two liquids (left) and an emulsion or rag layer between two liquids. Interface Between Liquids.
From present5.com
Surface Chemistry Toolkit Making sense of the role Interface Between Liquids While the term interfacial tension refers to interfaces between two liquids, the term surface tension refers to the interactions between a liquid and a gaseous phase. An example is the surface tension. A phase is an area in which physical quantities do not. This section addresses the case of interfaces between two fluids onto which a species insoluble in both. Interface Between Liquids.
From www.sciencephoto.com
Liquid Interface (1 of 2) Stock Image C028/0229 Science Photo Library Interface Between Liquids Modern level measurement technology is capable of detecting all kinds of interfaces, including multiple layers. This section addresses the case of interfaces between two fluids onto which a species insoluble in both of them has been. A clear interface between two liquids (left) and an emulsion or rag layer between two liquids (right). An interface is the contact plane between. Interface Between Liquids.
From www.chegg.com
Solved The drawings show two examples in which a ray of Interface Between Liquids An interface is the contact plane between two phases which do not mix. A clear interface between two liquids (left) and an emulsion or rag layer between two liquids (right). While the term interfacial tension refers to interfaces between two liquids, the term surface tension refers to the interactions between a liquid and a gaseous phase. This can be seen. Interface Between Liquids.
From blog.biolinscientific.com
What is surface tension? Interface Between Liquids Two basic kinds of level interfaces: A clear interface between two liquids (left) and an emulsion or rag layer between two liquids (right). An example is the surface tension. This can be seen if droplets of one liquid are dropped into the bulk. A phase is an area in which physical quantities do not. Modern level measurement technology is capable. Interface Between Liquids.
From ar.inspiredpencil.com
Water Molecules Liquid Interface Between Liquids An interface is the contact plane between two phases which do not mix. This section addresses the case of interfaces between two fluids onto which a species insoluble in both of them has been. This chapter examines the properties of interfaces between a liquid and a fluid (liquid or gas) containing multiple. While the term interfacial tension refers to interfaces. Interface Between Liquids.
From blog.isa.org
How to Measure the Interface Between Two Liquids in a Tank Interface Between Liquids Two basic kinds of level interfaces: An interface is the contact plane between two phases which do not mix. What is the difference between an interface and a surface? A clear interface between two liquids (left) and an emulsion or rag layer between two liquids (right). While the term interfacial tension refers to interfaces between two liquids, the term surface. Interface Between Liquids.
From www.researchgate.net
(a) Schematic of the solidliquid interface showing the interfacial Interface Between Liquids Two basic kinds of level interfaces: This section addresses the case of interfaces between two fluids onto which a species insoluble in both of them has been. A clear interface between two liquids (left) and an emulsion or rag layer between two liquids (right). This can be seen if droplets of one liquid are dropped into the bulk. An example. Interface Between Liquids.
From www.researchgate.net
Interface comparison of liquidliquid interface, liquidsolid Interface Between Liquids An interface is the contact plane between two phases which do not mix. What is the difference between an interface and a surface? Modern level measurement technology is capable of detecting all kinds of interfaces, including multiple layers. This section addresses the case of interfaces between two fluids onto which a species insoluble in both of them has been. This. Interface Between Liquids.
From slideplayer.com
Unit 9 States of Matter. ppt download Interface Between Liquids This chapter examines the properties of interfaces between a liquid and a fluid (liquid or gas) containing multiple. What is the difference between an interface and a surface? This can be seen if droplets of one liquid are dropped into the bulk. Modern level measurement technology is capable of detecting all kinds of interfaces, including multiple layers. This section addresses. Interface Between Liquids.
From pubs.rsc.org
Liquidliquid interfaces a unique and advantageous environment to Interface Between Liquids An example is the surface tension. An interface is the contact plane between two phases which do not mix. Two basic kinds of level interfaces: This section addresses the case of interfaces between two fluids onto which a species insoluble in both of them has been. This can be seen if droplets of one liquid are dropped into the bulk.. Interface Between Liquids.
From www.researchgate.net
Images of the gasliquid interfaces for three liquids at different Interface Between Liquids Two basic kinds of level interfaces: Modern level measurement technology is capable of detecting all kinds of interfaces, including multiple layers. An interface is the contact plane between two phases which do not mix. This chapter examines the properties of interfaces between a liquid and a fluid (liquid or gas) containing multiple. A clear interface between two liquids (left) and. Interface Between Liquids.
From www.slideserve.com
PPT LiquidGas and LiquidLiquid Interfaces (Chapter 4, pp. 64114 in Interface Between Liquids Two basic kinds of level interfaces: This section addresses the case of interfaces between two fluids onto which a species insoluble in both of them has been. This chapter examines the properties of interfaces between a liquid and a fluid (liquid or gas) containing multiple. This can be seen if droplets of one liquid are dropped into the bulk. While. Interface Between Liquids.
From pubs.acs.org
Liquid Heterostructures Generation of LiquidLiquid Interfaces in Free Interface Between Liquids An example is the surface tension. A phase is an area in which physical quantities do not. This section addresses the case of interfaces between two fluids onto which a species insoluble in both of them has been. This can be seen if droplets of one liquid are dropped into the bulk. What is the difference between an interface and. Interface Between Liquids.
From chem.libretexts.org
16.2 The Liquid State Chemistry LibreTexts Interface Between Liquids Two basic kinds of level interfaces: Modern level measurement technology is capable of detecting all kinds of interfaces, including multiple layers. This chapter examines the properties of interfaces between a liquid and a fluid (liquid or gas) containing multiple. An example is the surface tension. While the term interfacial tension refers to interfaces between two liquids, the term surface tension. Interface Between Liquids.
From www.slideserve.com
PPT LiquidGas and LiquidLiquid Interfaces (Chapter 4, pp. 64114 in Interface Between Liquids This chapter examines the properties of interfaces between a liquid and a fluid (liquid or gas) containing multiple. What is the difference between an interface and a surface? This can be seen if droplets of one liquid are dropped into the bulk. An interface is the contact plane between two phases which do not mix. Modern level measurement technology is. Interface Between Liquids.
From pubs.acs.org
Liquid Heterostructures Generation of LiquidLiquid Interfaces in Free Interface Between Liquids Modern level measurement technology is capable of detecting all kinds of interfaces, including multiple layers. This can be seen if droplets of one liquid are dropped into the bulk. A phase is an area in which physical quantities do not. Two basic kinds of level interfaces: While the term interfacial tension refers to interfaces between two liquids, the term surface. Interface Between Liquids.
From www.slideserve.com
PPT Liquid Interfaces Summary PowerPoint Presentation, free download Interface Between Liquids This section addresses the case of interfaces between two fluids onto which a species insoluble in both of them has been. While the term interfacial tension refers to interfaces between two liquids, the term surface tension refers to the interactions between a liquid and a gaseous phase. Modern level measurement technology is capable of detecting all kinds of interfaces, including. Interface Between Liquids.
From pubs.rsc.org
Liquidliquid interfaces a unique and advantageous environment to Interface Between Liquids This section addresses the case of interfaces between two fluids onto which a species insoluble in both of them has been. A phase is an area in which physical quantities do not. An example is the surface tension. What is the difference between an interface and a surface? A clear interface between two liquids (left) and an emulsion or rag. Interface Between Liquids.
From myloview.com
Interface between water and oil, two immiscible liquids, inside • wall Interface Between Liquids An interface is the contact plane between two phases which do not mix. Modern level measurement technology is capable of detecting all kinds of interfaces, including multiple layers. This chapter examines the properties of interfaces between a liquid and a fluid (liquid or gas) containing multiple. An example is the surface tension. What is the difference between an interface and. Interface Between Liquids.
From www.pnas.org
Fluid interfaces with very sharp tips in viscous flow PNAS Interface Between Liquids A clear interface between two liquids (left) and an emulsion or rag layer between two liquids (right). An example is the surface tension. This can be seen if droplets of one liquid are dropped into the bulk. A phase is an area in which physical quantities do not. What is the difference between an interface and a surface? Modern level. Interface Between Liquids.
From www.researchgate.net
Comparison of liquidvapor interface profiles between the present Interface Between Liquids Two basic kinds of level interfaces: An example is the surface tension. An interface is the contact plane between two phases which do not mix. Modern level measurement technology is capable of detecting all kinds of interfaces, including multiple layers. While the term interfacial tension refers to interfaces between two liquids, the term surface tension refers to the interactions between. Interface Between Liquids.
From www.researchgate.net
(a) Wavy interface between liquid and vapor layers [58]. (b Interface Between Liquids Two basic kinds of level interfaces: What is the difference between an interface and a surface? A clear interface between two liquids (left) and an emulsion or rag layer between two liquids (right). A phase is an area in which physical quantities do not. This can be seen if droplets of one liquid are dropped into the bulk. This section. Interface Between Liquids.
From www.researchgate.net
Model showing the liquid liquid interface (a) changing with manual Interface Between Liquids A phase is an area in which physical quantities do not. An example is the surface tension. An interface is the contact plane between two phases which do not mix. A clear interface between two liquids (left) and an emulsion or rag layer between two liquids (right). While the term interfacial tension refers to interfaces between two liquids, the term. Interface Between Liquids.
From researchoutreach.org
Facilitated ion transfer through liquidliquid interfaces Research Interface Between Liquids Modern level measurement technology is capable of detecting all kinds of interfaces, including multiple layers. Two basic kinds of level interfaces: This chapter examines the properties of interfaces between a liquid and a fluid (liquid or gas) containing multiple. This can be seen if droplets of one liquid are dropped into the bulk. An interface is the contact plane between. Interface Between Liquids.
From www.researchgate.net
(a) Xray reflectivity of the interface between liquid Hg and 0.01 M Interface Between Liquids While the term interfacial tension refers to interfaces between two liquids, the term surface tension refers to the interactions between a liquid and a gaseous phase. Modern level measurement technology is capable of detecting all kinds of interfaces, including multiple layers. This can be seen if droplets of one liquid are dropped into the bulk. An interface is the contact. Interface Between Liquids.
From www.chemicalprocessing.com
Accurately Measure Interfaces Between Immiscible Liquids Chemical Interface Between Liquids This chapter examines the properties of interfaces between a liquid and a fluid (liquid or gas) containing multiple. Two basic kinds of level interfaces: This section addresses the case of interfaces between two fluids onto which a species insoluble in both of them has been. A clear interface between two liquids (left) and an emulsion or rag layer between two. Interface Between Liquids.
From www.engineering.org.cn
HostGuest Molecular Recognition at LiquidLiquid Interfaces Interface Between Liquids A clear interface between two liquids (left) and an emulsion or rag layer between two liquids (right). While the term interfacial tension refers to interfaces between two liquids, the term surface tension refers to the interactions between a liquid and a gaseous phase. An example is the surface tension. Modern level measurement technology is capable of detecting all kinds of. Interface Between Liquids.
From www.youtube.com
Two Immiscible Fluids between Parallel Plates (Interactive) YouTube Interface Between Liquids A clear interface between two liquids (left) and an emulsion or rag layer between two liquids (right). What is the difference between an interface and a surface? Modern level measurement technology is capable of detecting all kinds of interfaces, including multiple layers. This chapter examines the properties of interfaces between a liquid and a fluid (liquid or gas) containing multiple.. Interface Between Liquids.
From www.youtube.com
EMA5001 L1108 Solid liquid interfaces YouTube Interface Between Liquids A clear interface between two liquids (left) and an emulsion or rag layer between two liquids (right). What is the difference between an interface and a surface? This section addresses the case of interfaces between two fluids onto which a species insoluble in both of them has been. While the term interfacial tension refers to interfaces between two liquids, the. Interface Between Liquids.