Zinc Chloride With Sodium Hydroxide . Place about 5cm 3 of the solution into a test tube. Zn(s) + 2hcl(aq) → zncl2(aq) +h2(g) bases. Sodium hydroxide + zinc chloride = zinc hydroxide + sodium chloride. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. The metal is more reactive than hydrogen and; Naoh + zncl2 = zn(oh)2 + nacl is a double displacement. Dissolve a small quantity of the substance in water. Acid + metal → salt + hydrogen. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. In effect zinc is not displacing sodium. Add a few drops of. We expect a metal to do this from a hydroxide base if.
from www.youtube.com
The metal is more reactive than hydrogen and; Acid + metal → salt + hydrogen. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. We expect a metal to do this from a hydroxide base if. Sodium hydroxide + zinc chloride = zinc hydroxide + sodium chloride. Add a few drops of. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Zn(s) + 2hcl(aq) → zncl2(aq) +h2(g) bases. In effect zinc is not displacing sodium. Place about 5cm 3 of the solution into a test tube.
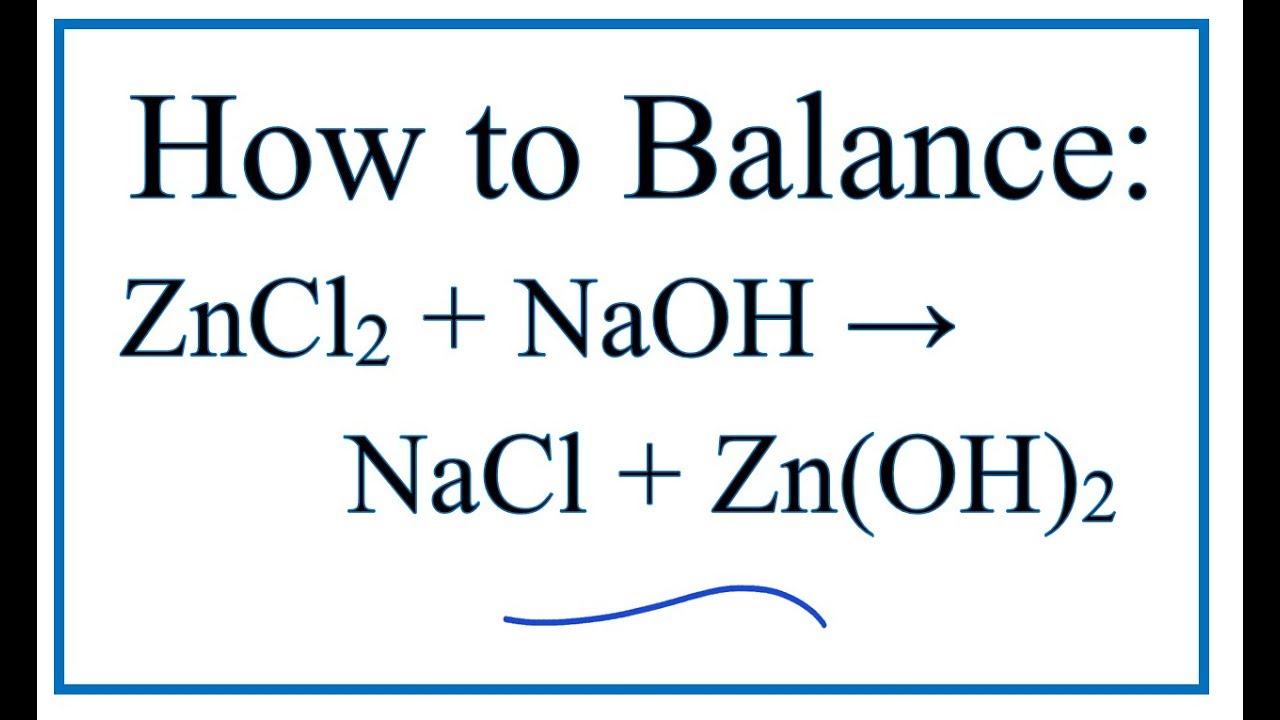
How to Balance ZnCl2 + NaOH = NaCl + Zn(OH)2 Zinc chloride + Sodium
Zinc Chloride With Sodium Hydroxide Naoh + zncl2 = zn(oh)2 + nacl is a double displacement. Sodium hydroxide + zinc chloride = zinc hydroxide + sodium chloride. Acid + metal → salt + hydrogen. Zn(s) + 2hcl(aq) → zncl2(aq) +h2(g) bases. Naoh + zncl2 = zn(oh)2 + nacl is a double displacement. We expect a metal to do this from a hydroxide base if. Dissolve a small quantity of the substance in water. In effect zinc is not displacing sodium. Place about 5cm 3 of the solution into a test tube. Add a few drops of. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. The metal is more reactive than hydrogen and; Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen.
From www.kayceechem.com
Zinc Chloride,Zinc Chloride (ZnCl2),Zinc Chloride Powder Manufacturers Zinc Chloride With Sodium Hydroxide We expect a metal to do this from a hydroxide base if. Naoh + zncl2 = zn(oh)2 + nacl is a double displacement. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. The metal is more reactive than hydrogen and; Add a few drops of. Place about 5cm 3 of the solution. Zinc Chloride With Sodium Hydroxide.
From www.researchgate.net
Comparison of Zn content in the precipitate after precipitation with Zinc Chloride With Sodium Hydroxide Dissolve a small quantity of the substance in water. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Acid + metal → salt + hydrogen. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Sodium hydroxide + zinc chloride = zinc hydroxide + sodium chloride. We expect. Zinc Chloride With Sodium Hydroxide.
From www.numerade.com
SOLVED Write an unbalanced equation to represent each of the following Zinc Chloride With Sodium Hydroxide The metal is more reactive than hydrogen and; We expect a metal to do this from a hydroxide base if. Acid + metal → salt + hydrogen. Sodium hydroxide + zinc chloride = zinc hydroxide + sodium chloride. Place about 5cm 3 of the solution into a test tube. In effect zinc is not displacing sodium. Add a few drops. Zinc Chloride With Sodium Hydroxide.
From www.sciencephoto.com
Sodium hydroxide and transition metals Stock Image C028/4198 Zinc Chloride With Sodium Hydroxide Add a few drops of. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. The metal is more reactive than hydrogen and; In effect zinc is not displacing sodium. Naoh + zncl2 = zn(oh)2 + nacl is a double displacement. Acid + metal → salt + hydrogen. Zn(s) + 2hcl(aq) → zncl2(aq) +h2(g) bases. Sodium. Zinc Chloride With Sodium Hydroxide.
From www.gkseries.com
When sodium hydroxide reacts with Zinc it produces Zinc Chloride With Sodium Hydroxide We expect a metal to do this from a hydroxide base if. In effect zinc is not displacing sodium. Add a few drops of. Place about 5cm 3 of the solution into a test tube. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Zn(s) + 2hcl(aq) → zncl2(aq) +h2(g) bases. Acid. Zinc Chloride With Sodium Hydroxide.
From fyohdfutv.blob.core.windows.net
Zinc Chloride And Sodium Hydroxide at John Hauser blog Zinc Chloride With Sodium Hydroxide Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. The metal is more reactive than hydrogen and; Sodium hydroxide + zinc chloride = zinc hydroxide + sodium chloride. Dissolve a small quantity of the substance in water. Acid + metal → salt + hydrogen. We expect a metal to do this from. Zinc Chloride With Sodium Hydroxide.
From pediaa.com
Difference Between Deliquescent Efflorescent and Hygroscopic Zinc Chloride With Sodium Hydroxide Place about 5cm 3 of the solution into a test tube. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Sodium hydroxide + zinc chloride = zinc hydroxide + sodium chloride. Add a few drops of. The metal is more reactive than hydrogen and; We expect a metal to do this from a hydroxide base. Zinc Chloride With Sodium Hydroxide.
From www.hanqi.com.cn
Basic zinc chloride or Zinc chloride hydroxide ZINC HANQI INDUSTRY Zinc Chloride With Sodium Hydroxide Place about 5cm 3 of the solution into a test tube. Naoh + zncl2 = zn(oh)2 + nacl is a double displacement. The metal is more reactive than hydrogen and; Acid + metal → salt + hydrogen. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Add a few drops of. In effect zinc is. Zinc Chloride With Sodium Hydroxide.
From www.gkseries.com
What is formed when zinc reacts with sodium hydroxide? Zinc Chloride With Sodium Hydroxide Naoh + zncl2 = zn(oh)2 + nacl is a double displacement. The metal is more reactive than hydrogen and; Sodium hydroxide + zinc chloride = zinc hydroxide + sodium chloride. We expect a metal to do this from a hydroxide base if. Dissolve a small quantity of the substance in water. In effect zinc is not displacing sodium. For example,. Zinc Chloride With Sodium Hydroxide.
From www.researchgate.net
Synthesis of zinc oxide nanoparticles using zinc chloride and sodium Zinc Chloride With Sodium Hydroxide Naoh + zncl2 = zn(oh)2 + nacl is a double displacement. We expect a metal to do this from a hydroxide base if. Add a few drops of. Dissolve a small quantity of the substance in water. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. For example, zinc metal reacts with. Zinc Chloride With Sodium Hydroxide.
From www.flinnsci.com
Flinn Chemicals, Zinc Chloride Zinc Chloride With Sodium Hydroxide Sodium hydroxide + zinc chloride = zinc hydroxide + sodium chloride. The metal is more reactive than hydrogen and; In effect zinc is not displacing sodium. Acid + metal → salt + hydrogen. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Dissolve a small quantity of the substance in water. Acids will react with. Zinc Chloride With Sodium Hydroxide.
From camachem.com
Industrial Chemicals for Sale Zinc Chloride 98 Contact CAMACHEM Zinc Chloride With Sodium Hydroxide Acid + metal → salt + hydrogen. The metal is more reactive than hydrogen and; We expect a metal to do this from a hydroxide base if. Naoh + zncl2 = zn(oh)2 + nacl is a double displacement. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Dissolve a small quantity of. Zinc Chloride With Sodium Hydroxide.
From brainly.in
zinc is put in concentrated sodium hydroxide. explain with the help of Zinc Chloride With Sodium Hydroxide Dissolve a small quantity of the substance in water. We expect a metal to do this from a hydroxide base if. In effect zinc is not displacing sodium. Naoh + zncl2 = zn(oh)2 + nacl is a double displacement. The metal is more reactive than hydrogen and; Acids will react with reactive metals, such as magnesium and zinc, to make. Zinc Chloride With Sodium Hydroxide.
From cartoondealer.com
Sodium Hydroxide Pellets, Zinc Granules And Copper(II) Sulfate In Test Zinc Chloride With Sodium Hydroxide Sodium hydroxide + zinc chloride = zinc hydroxide + sodium chloride. Acid + metal → salt + hydrogen. Zn(s) + 2hcl(aq) → zncl2(aq) +h2(g) bases. Dissolve a small quantity of the substance in water. Add a few drops of. Place about 5cm 3 of the solution into a test tube. Acids will react with reactive metals, such as magnesium and. Zinc Chloride With Sodium Hydroxide.
From fineartamerica.com
Zinc Hydroxide Precipitate Photograph by Andrew Lambert Photography Zinc Chloride With Sodium Hydroxide The metal is more reactive than hydrogen and; Dissolve a small quantity of the substance in water. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Sodium hydroxide + zinc chloride = zinc hydroxide + sodium chloride. Zn(s) + 2hcl(aq) → zncl2(aq) +h2(g) bases. For example, zinc metal reacts with hydrochloric acid,. Zinc Chloride With Sodium Hydroxide.
From www.youtube.com
How to Balance ZnCl2 + NaOH = NaCl + Zn(OH)2 Zinc chloride + Sodium Zinc Chloride With Sodium Hydroxide The metal is more reactive than hydrogen and; For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. In effect zinc is not displacing sodium. Sodium hydroxide + zinc chloride = zinc hydroxide + sodium chloride. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Dissolve a small. Zinc Chloride With Sodium Hydroxide.
From www.dreamstime.com
Molecular Formula of Zinc Chloride Stock Illustration Illustration of Zinc Chloride With Sodium Hydroxide The metal is more reactive than hydrogen and; Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Acid + metal → salt + hydrogen. Dissolve a small quantity of the substance in water. We expect a metal to do this from a hydroxide base if. In effect zinc is not displacing sodium.. Zinc Chloride With Sodium Hydroxide.
From fphoto.photoshelter.com
science chemistry redox reaction zinc hydrochloric acid Fundamental Zinc Chloride With Sodium Hydroxide Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Naoh + zncl2 = zn(oh)2 + nacl is a double displacement. We expect a metal to do this from a hydroxide base if. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Zn(s) + 2hcl(aq) → zncl2(aq) +h2(g). Zinc Chloride With Sodium Hydroxide.
From www.researchgate.net
Reagents and conditions. (a) Ammonium chloride, zinc acetate, sodium Zinc Chloride With Sodium Hydroxide We expect a metal to do this from a hydroxide base if. Sodium hydroxide + zinc chloride = zinc hydroxide + sodium chloride. Acid + metal → salt + hydrogen. Naoh + zncl2 = zn(oh)2 + nacl is a double displacement. Dissolve a small quantity of the substance in water. Zn(s) + 2hcl(aq) → zncl2(aq) +h2(g) bases. For example, zinc. Zinc Chloride With Sodium Hydroxide.
From niurichem.en.made-in-china.com
Soluble in Acid Sodium Hydroxide Industrial Oxidizer Zinc Oxide China Zinc Chloride With Sodium Hydroxide Naoh + zncl2 = zn(oh)2 + nacl is a double displacement. The metal is more reactive than hydrogen and; Zn(s) + 2hcl(aq) → zncl2(aq) +h2(g) bases. Sodium hydroxide + zinc chloride = zinc hydroxide + sodium chloride. Dissolve a small quantity of the substance in water. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas.. Zinc Chloride With Sodium Hydroxide.
From www.sigmaaldrich.co.th
ZINC CHLORIDE, 98+ Merck Life Sciences Thailand Zinc Chloride With Sodium Hydroxide Acid + metal → salt + hydrogen. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. We expect a metal to do this from a hydroxide base if. Dissolve a small quantity of the substance in water. In effect zinc is not displacing sodium. Naoh + zncl2 = zn(oh)2 + nacl is. Zinc Chloride With Sodium Hydroxide.
From alchetron.com
Zinc chloride hydroxide monohydrate Alchetron, the free social Zinc Chloride With Sodium Hydroxide Sodium hydroxide + zinc chloride = zinc hydroxide + sodium chloride. Naoh + zncl2 = zn(oh)2 + nacl is a double displacement. In effect zinc is not displacing sodium. Dissolve a small quantity of the substance in water. Acid + metal → salt + hydrogen. Place about 5cm 3 of the solution into a test tube. We expect a metal. Zinc Chloride With Sodium Hydroxide.
From www.scielo.br
SciELO Brasil Synthesis and Characterization of Zinc Oxide Obtained Zinc Chloride With Sodium Hydroxide Zn(s) + 2hcl(aq) → zncl2(aq) +h2(g) bases. Acid + metal → salt + hydrogen. Naoh + zncl2 = zn(oh)2 + nacl is a double displacement. In effect zinc is not displacing sodium. Place about 5cm 3 of the solution into a test tube. Sodium hydroxide + zinc chloride = zinc hydroxide + sodium chloride. Dissolve a small quantity of the. Zinc Chloride With Sodium Hydroxide.
From www.indiamart.com
250 g Zinc Chloride Dry at best price in Pinjore ID 23826350562 Zinc Chloride With Sodium Hydroxide Add a few drops of. The metal is more reactive than hydrogen and; Sodium hydroxide + zinc chloride = zinc hydroxide + sodium chloride. Zn(s) + 2hcl(aq) → zncl2(aq) +h2(g) bases. Acid + metal → salt + hydrogen. In effect zinc is not displacing sodium. Naoh + zncl2 = zn(oh)2 + nacl is a double displacement. We expect a metal. Zinc Chloride With Sodium Hydroxide.
From www.sciencephoto.com
Zinc Hydroxide Precipitate Stock Image C027/9442 Science Photo Zinc Chloride With Sodium Hydroxide The metal is more reactive than hydrogen and; Place about 5cm 3 of the solution into a test tube. In effect zinc is not displacing sodium. Add a few drops of. Naoh + zncl2 = zn(oh)2 + nacl is a double displacement. We expect a metal to do this from a hydroxide base if. Zn(s) + 2hcl(aq) → zncl2(aq) +h2(g). Zinc Chloride With Sodium Hydroxide.
From www.youtube.com
What happens when Sodium hydroxide (NaOH) reacts with zinc chloride Zinc Chloride With Sodium Hydroxide We expect a metal to do this from a hydroxide base if. In effect zinc is not displacing sodium. Add a few drops of. Acid + metal → salt + hydrogen. Dissolve a small quantity of the substance in water. Naoh + zncl2 = zn(oh)2 + nacl is a double displacement. For example, zinc metal reacts with hydrochloric acid, producing. Zinc Chloride With Sodium Hydroxide.
From www.dreamstime.com
ZnCl2 Zinc chloride stock photo. Image of bottle, glass 288820052 Zinc Chloride With Sodium Hydroxide Add a few drops of. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Sodium hydroxide + zinc chloride = zinc hydroxide + sodium chloride. Dissolve a small quantity of the substance in water. Naoh + zncl2 = zn(oh)2 + nacl is a double displacement. We expect a metal to do this from a hydroxide. Zinc Chloride With Sodium Hydroxide.
From www.pw.live
Zinc Chloride Formula Zinc Chloride With Sodium Hydroxide Zn(s) + 2hcl(aq) → zncl2(aq) +h2(g) bases. Add a few drops of. Naoh + zncl2 = zn(oh)2 + nacl is a double displacement. We expect a metal to do this from a hydroxide base if. The metal is more reactive than hydrogen and; Sodium hydroxide + zinc chloride = zinc hydroxide + sodium chloride. Acid + metal → salt +. Zinc Chloride With Sodium Hydroxide.
From www.youtube.com
Cation Test Zinc ion with Sodium Hydroxide YouTube Zinc Chloride With Sodium Hydroxide Sodium hydroxide + zinc chloride = zinc hydroxide + sodium chloride. Zn(s) + 2hcl(aq) → zncl2(aq) +h2(g) bases. The metal is more reactive than hydrogen and; In effect zinc is not displacing sodium. Add a few drops of. We expect a metal to do this from a hydroxide base if. For example, zinc metal reacts with hydrochloric acid, producing zinc. Zinc Chloride With Sodium Hydroxide.
From ar.inspiredpencil.com
Zinc Hydroxide Zinc Chloride With Sodium Hydroxide Naoh + zncl2 = zn(oh)2 + nacl is a double displacement. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Place about 5cm 3 of the solution into a test tube. In effect zinc is not displacing sodium. Add a few drops of. Dissolve a small quantity of the substance in water. Acids will react. Zinc Chloride With Sodium Hydroxide.
From www.slideserve.com
PPT DO NOW!! PowerPoint Presentation, free download ID1560113 Zinc Chloride With Sodium Hydroxide The metal is more reactive than hydrogen and; In effect zinc is not displacing sodium. Dissolve a small quantity of the substance in water. We expect a metal to do this from a hydroxide base if. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Zn(s) + 2hcl(aq) → zncl2(aq) +h2(g) bases. Add a few. Zinc Chloride With Sodium Hydroxide.
From www.alamy.com
Zinc hydroxide precipitate formed by adding sodium hydroxide (NaOH) to Zinc Chloride With Sodium Hydroxide Dissolve a small quantity of the substance in water. Zn(s) + 2hcl(aq) → zncl2(aq) +h2(g) bases. Place about 5cm 3 of the solution into a test tube. We expect a metal to do this from a hydroxide base if. Add a few drops of. Naoh + zncl2 = zn(oh)2 + nacl is a double displacement. In effect zinc is not. Zinc Chloride With Sodium Hydroxide.
From www.youtube.com
QA Test for Zinc Ion with aqueous sodium hydroxide YouTube Zinc Chloride With Sodium Hydroxide Zn(s) + 2hcl(aq) → zncl2(aq) +h2(g) bases. The metal is more reactive than hydrogen and; Add a few drops of. Dissolve a small quantity of the substance in water. We expect a metal to do this from a hydroxide base if. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. In effect. Zinc Chloride With Sodium Hydroxide.
From www.youtube.com
How to Write the Net Ionic Equation for ZnCl2 + NaOH = Zn(OH)2 + NaCl Zinc Chloride With Sodium Hydroxide We expect a metal to do this from a hydroxide base if. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. The metal is more reactive than hydrogen and; Add a few drops of. Acid + metal → salt + hydrogen. Zn(s) + 2hcl(aq) → zncl2(aq) +h2(g) bases. In effect zinc is. Zinc Chloride With Sodium Hydroxide.
From www.shutterstock.com
122 Zinc Chloride Images, Stock Photos & Vectors Shutterstock Zinc Chloride With Sodium Hydroxide Place about 5cm 3 of the solution into a test tube. Acid + metal → salt + hydrogen. In effect zinc is not displacing sodium. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. We expect a metal to do this from a hydroxide base if. Add a few drops of. For. Zinc Chloride With Sodium Hydroxide.