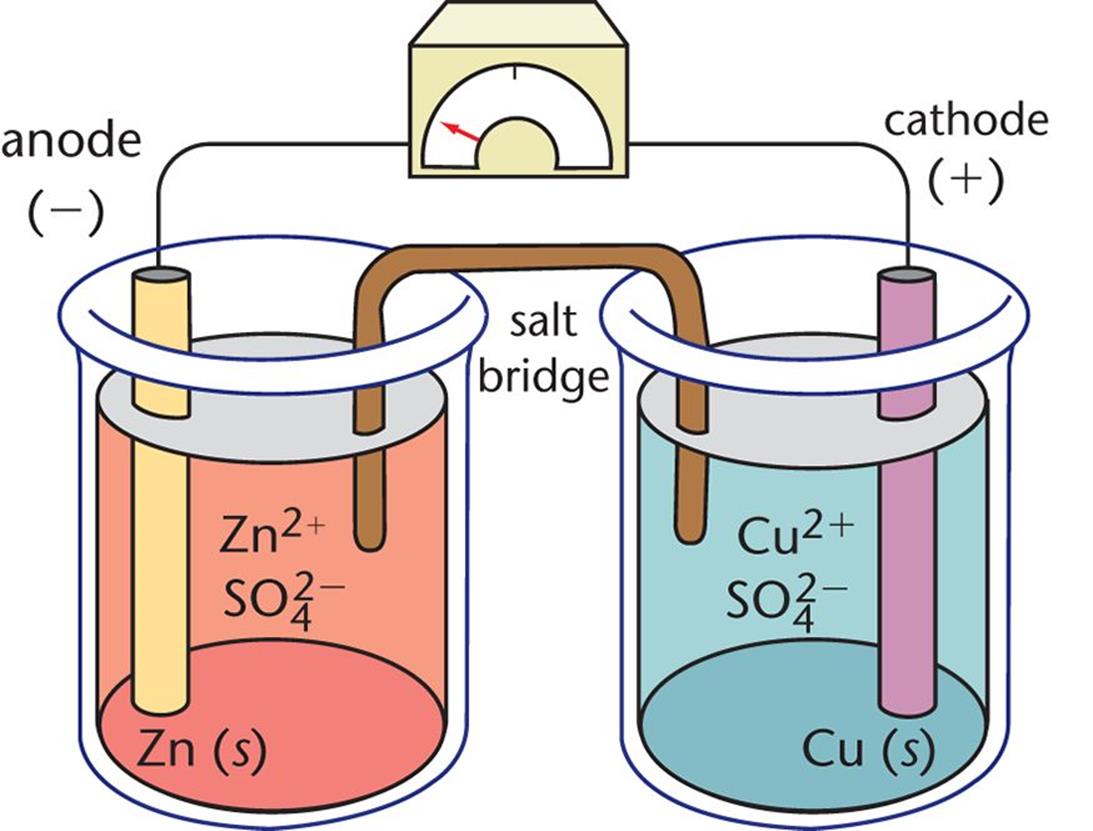
Electrochemistry Galvanic Cells Lab . Use the nernst equation to calculate the concentration. A voltaic cell, also known as a galvanic cell, is an electrochemical cell where a spontaneous reaction generates an electrical current. In the laboratory, a typical electrochemical cell has the following general construction: Predict the voltage for galvanic cells using standard reduction potentials. Vary concentrations of one solution in.
from klaefpten.blob.core.windows.net
In the laboratory, a typical electrochemical cell has the following general construction: Predict the voltage for galvanic cells using standard reduction potentials. Use the nernst equation to calculate the concentration. A voltaic cell, also known as a galvanic cell, is an electrochemical cell where a spontaneous reaction generates an electrical current. Vary concentrations of one solution in.
Electrochemical And Electrolytic Cell Comparison at Luther Estes blog
Electrochemistry Galvanic Cells Lab In the laboratory, a typical electrochemical cell has the following general construction: A voltaic cell, also known as a galvanic cell, is an electrochemical cell where a spontaneous reaction generates an electrical current. Vary concentrations of one solution in. Predict the voltage for galvanic cells using standard reduction potentials. In the laboratory, a typical electrochemical cell has the following general construction: Use the nernst equation to calculate the concentration.
From study.com
Electrochemical Cell Definition, Types & Examples Lesson Electrochemistry Galvanic Cells Lab Vary concentrations of one solution in. A voltaic cell, also known as a galvanic cell, is an electrochemical cell where a spontaneous reaction generates an electrical current. Predict the voltage for galvanic cells using standard reduction potentials. Use the nernst equation to calculate the concentration. In the laboratory, a typical electrochemical cell has the following general construction: Electrochemistry Galvanic Cells Lab.
From ar.inspiredpencil.com
Blank Galvanic Cell Electrochemistry Galvanic Cells Lab Use the nernst equation to calculate the concentration. Vary concentrations of one solution in. In the laboratory, a typical electrochemical cell has the following general construction: A voltaic cell, also known as a galvanic cell, is an electrochemical cell where a spontaneous reaction generates an electrical current. Predict the voltage for galvanic cells using standard reduction potentials. Electrochemistry Galvanic Cells Lab.
From chem.libretexts.org
1 Electrochemical Cells (Experiment) Chemistry LibreTexts Electrochemistry Galvanic Cells Lab Predict the voltage for galvanic cells using standard reduction potentials. Vary concentrations of one solution in. In the laboratory, a typical electrochemical cell has the following general construction: Use the nernst equation to calculate the concentration. A voltaic cell, also known as a galvanic cell, is an electrochemical cell where a spontaneous reaction generates an electrical current. Electrochemistry Galvanic Cells Lab.
From learningkurugon1.z22.web.core.windows.net
Electrochemical Cells Gcse Chemistry Electrochemistry Galvanic Cells Lab Predict the voltage for galvanic cells using standard reduction potentials. Vary concentrations of one solution in. A voltaic cell, also known as a galvanic cell, is an electrochemical cell where a spontaneous reaction generates an electrical current. Use the nernst equation to calculate the concentration. In the laboratory, a typical electrochemical cell has the following general construction: Electrochemistry Galvanic Cells Lab.
From kladwjlgf.blob.core.windows.net
Electrochemistry Galvanic Cells at Roberto Alvarado blog Electrochemistry Galvanic Cells Lab Predict the voltage for galvanic cells using standard reduction potentials. Vary concentrations of one solution in. In the laboratory, a typical electrochemical cell has the following general construction: A voltaic cell, also known as a galvanic cell, is an electrochemical cell where a spontaneous reaction generates an electrical current. Use the nernst equation to calculate the concentration. Electrochemistry Galvanic Cells Lab.
From ar.inspiredpencil.com
Galvanic Cell Vs Electrolytic Cell Electrochemistry Galvanic Cells Lab Predict the voltage for galvanic cells using standard reduction potentials. Vary concentrations of one solution in. Use the nernst equation to calculate the concentration. A voltaic cell, also known as a galvanic cell, is an electrochemical cell where a spontaneous reaction generates an electrical current. In the laboratory, a typical electrochemical cell has the following general construction: Electrochemistry Galvanic Cells Lab.
From www.studocu.com
Chap 19 pt2 Galvanic Cells intro Chapter 19 Electrochemistry Electrochemistry Galvanic Cells Lab A voltaic cell, also known as a galvanic cell, is an electrochemical cell where a spontaneous reaction generates an electrical current. Vary concentrations of one solution in. In the laboratory, a typical electrochemical cell has the following general construction: Predict the voltage for galvanic cells using standard reduction potentials. Use the nernst equation to calculate the concentration. Electrochemistry Galvanic Cells Lab.
From www.pinterest.com
Science Clipart and Diagrams Galvanic cell, Science clipart Electrochemistry Galvanic Cells Lab A voltaic cell, also known as a galvanic cell, is an electrochemical cell where a spontaneous reaction generates an electrical current. In the laboratory, a typical electrochemical cell has the following general construction: Use the nernst equation to calculate the concentration. Predict the voltage for galvanic cells using standard reduction potentials. Vary concentrations of one solution in. Electrochemistry Galvanic Cells Lab.
From studylib.net
1 ELECTROCHEMICAL CELLS LAB Purpose The purpose of this Electrochemistry Galvanic Cells Lab Predict the voltage for galvanic cells using standard reduction potentials. Use the nernst equation to calculate the concentration. A voltaic cell, also known as a galvanic cell, is an electrochemical cell where a spontaneous reaction generates an electrical current. Vary concentrations of one solution in. In the laboratory, a typical electrochemical cell has the following general construction: Electrochemistry Galvanic Cells Lab.
From klaefpten.blob.core.windows.net
Electrochemical And Electrolytic Cell Comparison at Luther Estes blog Electrochemistry Galvanic Cells Lab Predict the voltage for galvanic cells using standard reduction potentials. In the laboratory, a typical electrochemical cell has the following general construction: Vary concentrations of one solution in. A voltaic cell, also known as a galvanic cell, is an electrochemical cell where a spontaneous reaction generates an electrical current. Use the nernst equation to calculate the concentration. Electrochemistry Galvanic Cells Lab.
From employees.csbsju.edu
CHEM 123 GENERAL CHEMISTRY 1 Electrochemistry Galvanic Cells Lab Predict the voltage for galvanic cells using standard reduction potentials. In the laboratory, a typical electrochemical cell has the following general construction: Use the nernst equation to calculate the concentration. A voltaic cell, also known as a galvanic cell, is an electrochemical cell where a spontaneous reaction generates an electrical current. Vary concentrations of one solution in. Electrochemistry Galvanic Cells Lab.
From chem.libretexts.org
Voltaic Cells Chemistry LibreTexts Electrochemistry Galvanic Cells Lab A voltaic cell, also known as a galvanic cell, is an electrochemical cell where a spontaneous reaction generates an electrical current. In the laboratory, a typical electrochemical cell has the following general construction: Predict the voltage for galvanic cells using standard reduction potentials. Use the nernst equation to calculate the concentration. Vary concentrations of one solution in. Electrochemistry Galvanic Cells Lab.
From www.studocu.com
Galvanic Cell Lab Notes Electrochemistry Galvanic Cell Lab Electrochemistry Galvanic Cells Lab In the laboratory, a typical electrochemical cell has the following general construction: Vary concentrations of one solution in. A voltaic cell, also known as a galvanic cell, is an electrochemical cell where a spontaneous reaction generates an electrical current. Use the nernst equation to calculate the concentration. Predict the voltage for galvanic cells using standard reduction potentials. Electrochemistry Galvanic Cells Lab.
From www.studocu.com
Electrochemical cellsreport Investigating Electrochemical cells Aim Electrochemistry Galvanic Cells Lab In the laboratory, a typical electrochemical cell has the following general construction: Use the nernst equation to calculate the concentration. Vary concentrations of one solution in. A voltaic cell, also known as a galvanic cell, is an electrochemical cell where a spontaneous reaction generates an electrical current. Predict the voltage for galvanic cells using standard reduction potentials. Electrochemistry Galvanic Cells Lab.
From saylordotorg.github.io
Describing Electrochemical Cells Electrochemistry Galvanic Cells Lab Vary concentrations of one solution in. Predict the voltage for galvanic cells using standard reduction potentials. A voltaic cell, also known as a galvanic cell, is an electrochemical cell where a spontaneous reaction generates an electrical current. In the laboratory, a typical electrochemical cell has the following general construction: Use the nernst equation to calculate the concentration. Electrochemistry Galvanic Cells Lab.
From www1.chem.umn.edu
CopperZinc Galvanic Cell Electrochemistry Galvanic Cells Lab Use the nernst equation to calculate the concentration. A voltaic cell, also known as a galvanic cell, is an electrochemical cell where a spontaneous reaction generates an electrical current. Vary concentrations of one solution in. Predict the voltage for galvanic cells using standard reduction potentials. In the laboratory, a typical electrochemical cell has the following general construction: Electrochemistry Galvanic Cells Lab.
From greenenergymaterial.com
Basics Of Electrochemical Cells » Green Energy Material Electrochemistry Galvanic Cells Lab Vary concentrations of one solution in. Use the nernst equation to calculate the concentration. Predict the voltage for galvanic cells using standard reduction potentials. In the laboratory, a typical electrochemical cell has the following general construction: A voltaic cell, also known as a galvanic cell, is an electrochemical cell where a spontaneous reaction generates an electrical current. Electrochemistry Galvanic Cells Lab.
From courses.lumenlearning.com
Galvanic Cells Chemistry Electrochemistry Galvanic Cells Lab Use the nernst equation to calculate the concentration. A voltaic cell, also known as a galvanic cell, is an electrochemical cell where a spontaneous reaction generates an electrical current. Vary concentrations of one solution in. Predict the voltage for galvanic cells using standard reduction potentials. In the laboratory, a typical electrochemical cell has the following general construction: Electrochemistry Galvanic Cells Lab.
From www.studypool.com
SOLUTION Electrochemistry Galvanic Cells Lab Report Studypool Electrochemistry Galvanic Cells Lab A voltaic cell, also known as a galvanic cell, is an electrochemical cell where a spontaneous reaction generates an electrical current. Predict the voltage for galvanic cells using standard reduction potentials. Vary concentrations of one solution in. In the laboratory, a typical electrochemical cell has the following general construction: Use the nernst equation to calculate the concentration. Electrochemistry Galvanic Cells Lab.
From www.numerade.com
SOLVED Electrochemistry Galvanic Cells Data Sheet Name [Insert Name Electrochemistry Galvanic Cells Lab Use the nernst equation to calculate the concentration. In the laboratory, a typical electrochemical cell has the following general construction: Vary concentrations of one solution in. A voltaic cell, also known as a galvanic cell, is an electrochemical cell where a spontaneous reaction generates an electrical current. Predict the voltage for galvanic cells using standard reduction potentials. Electrochemistry Galvanic Cells Lab.
From www.youtube.com
Chem Lab Galvanic Cell /Electrochemical Cell, Voltmeter and Salt Electrochemistry Galvanic Cells Lab A voltaic cell, also known as a galvanic cell, is an electrochemical cell where a spontaneous reaction generates an electrical current. Vary concentrations of one solution in. Use the nernst equation to calculate the concentration. Predict the voltage for galvanic cells using standard reduction potentials. In the laboratory, a typical electrochemical cell has the following general construction: Electrochemistry Galvanic Cells Lab.
From worksheetmagicsantos.z13.web.core.windows.net
Electrochemical Cells A Level Chemistry Electrochemistry Galvanic Cells Lab Vary concentrations of one solution in. Predict the voltage for galvanic cells using standard reduction potentials. In the laboratory, a typical electrochemical cell has the following general construction: Use the nernst equation to calculate the concentration. A voltaic cell, also known as a galvanic cell, is an electrochemical cell where a spontaneous reaction generates an electrical current. Electrochemistry Galvanic Cells Lab.
From wisc.pb.unizin.org
Day 39 Voltaic Cells Chemistry 109 Electrochemistry Galvanic Cells Lab Use the nernst equation to calculate the concentration. Vary concentrations of one solution in. Predict the voltage for galvanic cells using standard reduction potentials. In the laboratory, a typical electrochemical cell has the following general construction: A voltaic cell, also known as a galvanic cell, is an electrochemical cell where a spontaneous reaction generates an electrical current. Electrochemistry Galvanic Cells Lab.
From www.scienceabc.com
What Are Galvanic Cells? An Oversimplified Explanation » ScienceABC Electrochemistry Galvanic Cells Lab Vary concentrations of one solution in. Use the nernst equation to calculate the concentration. In the laboratory, a typical electrochemical cell has the following general construction: Predict the voltage for galvanic cells using standard reduction potentials. A voltaic cell, also known as a galvanic cell, is an electrochemical cell where a spontaneous reaction generates an electrical current. Electrochemistry Galvanic Cells Lab.
From www.vecteezy.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and Electrochemistry Galvanic Cells Lab Predict the voltage for galvanic cells using standard reduction potentials. Vary concentrations of one solution in. Use the nernst equation to calculate the concentration. In the laboratory, a typical electrochemical cell has the following general construction: A voltaic cell, also known as a galvanic cell, is an electrochemical cell where a spontaneous reaction generates an electrical current. Electrochemistry Galvanic Cells Lab.
From www.studocu.com
Electrochemistry Lab Investing Galvanic Cells Investigating Galvanic Electrochemistry Galvanic Cells Lab A voltaic cell, also known as a galvanic cell, is an electrochemical cell where a spontaneous reaction generates an electrical current. Use the nernst equation to calculate the concentration. Vary concentrations of one solution in. Predict the voltage for galvanic cells using standard reduction potentials. In the laboratory, a typical electrochemical cell has the following general construction: Electrochemistry Galvanic Cells Lab.
From leah4sci.com
Electrochemistry Galvanic / Voltaic and Electrolytic Cells MCAT and Electrochemistry Galvanic Cells Lab Vary concentrations of one solution in. Use the nernst equation to calculate the concentration. A voltaic cell, also known as a galvanic cell, is an electrochemical cell where a spontaneous reaction generates an electrical current. Predict the voltage for galvanic cells using standard reduction potentials. In the laboratory, a typical electrochemical cell has the following general construction: Electrochemistry Galvanic Cells Lab.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Electrochemistry Galvanic Cells Lab Use the nernst equation to calculate the concentration. Vary concentrations of one solution in. Predict the voltage for galvanic cells using standard reduction potentials. In the laboratory, a typical electrochemical cell has the following general construction: A voltaic cell, also known as a galvanic cell, is an electrochemical cell where a spontaneous reaction generates an electrical current. Electrochemistry Galvanic Cells Lab.
From courses.lumenlearning.com
Galvanic Cells General Chemistry Electrochemistry Galvanic Cells Lab In the laboratory, a typical electrochemical cell has the following general construction: Predict the voltage for galvanic cells using standard reduction potentials. A voltaic cell, also known as a galvanic cell, is an electrochemical cell where a spontaneous reaction generates an electrical current. Use the nernst equation to calculate the concentration. Vary concentrations of one solution in. Electrochemistry Galvanic Cells Lab.
From www.pinterest.com
20.3 Voltaic Cells Galvanic cell, Chemistry experiments Electrochemistry Galvanic Cells Lab A voltaic cell, also known as a galvanic cell, is an electrochemical cell where a spontaneous reaction generates an electrical current. Use the nernst equation to calculate the concentration. Predict the voltage for galvanic cells using standard reduction potentials. Vary concentrations of one solution in. In the laboratory, a typical electrochemical cell has the following general construction: Electrochemistry Galvanic Cells Lab.
From studiousguy.com
Examples of Galvanic Cells in Everyday Life StudiousGuy Electrochemistry Galvanic Cells Lab Vary concentrations of one solution in. In the laboratory, a typical electrochemical cell has the following general construction: A voltaic cell, also known as a galvanic cell, is an electrochemical cell where a spontaneous reaction generates an electrical current. Predict the voltage for galvanic cells using standard reduction potentials. Use the nernst equation to calculate the concentration. Electrochemistry Galvanic Cells Lab.
From socratic.org
How can a galvanic cell an electrolytic cell? Socratic Electrochemistry Galvanic Cells Lab A voltaic cell, also known as a galvanic cell, is an electrochemical cell where a spontaneous reaction generates an electrical current. Use the nernst equation to calculate the concentration. Vary concentrations of one solution in. Predict the voltage for galvanic cells using standard reduction potentials. In the laboratory, a typical electrochemical cell has the following general construction: Electrochemistry Galvanic Cells Lab.
From www.alamy.com
Electrochemical cell or Galvanic cell, The Daniell cell with Voltmeter Electrochemistry Galvanic Cells Lab Vary concentrations of one solution in. Predict the voltage for galvanic cells using standard reduction potentials. A voltaic cell, also known as a galvanic cell, is an electrochemical cell where a spontaneous reaction generates an electrical current. Use the nernst equation to calculate the concentration. In the laboratory, a typical electrochemical cell has the following general construction: Electrochemistry Galvanic Cells Lab.
From classnotes.org.in
Electrochemical Cells Chemistry, Class 12, Electro Chemistry Electrochemistry Galvanic Cells Lab A voltaic cell, also known as a galvanic cell, is an electrochemical cell where a spontaneous reaction generates an electrical current. Predict the voltage for galvanic cells using standard reduction potentials. In the laboratory, a typical electrochemical cell has the following general construction: Vary concentrations of one solution in. Use the nernst equation to calculate the concentration. Electrochemistry Galvanic Cells Lab.
From saylordotorg.github.io
Electrochemistry Electrochemistry Galvanic Cells Lab Use the nernst equation to calculate the concentration. Vary concentrations of one solution in. Predict the voltage for galvanic cells using standard reduction potentials. In the laboratory, a typical electrochemical cell has the following general construction: A voltaic cell, also known as a galvanic cell, is an electrochemical cell where a spontaneous reaction generates an electrical current. Electrochemistry Galvanic Cells Lab.