Is Methanal An Aldehyde . Methanal (formaldehyde, hcho) colourless, inflammable,. The stem names of aldehydes and ketones are derived from those of the parent alkanes, defined by the longest continuous chain (lcc) of carbon atoms that contains the functional group. Views 2,215,066 updated may 11 2018. Where aldehydes and ketones differ. The stem names of aldehydes and ketones are derived from those of the parent alkanes, defined by the longest continuous chain (lcc) of carbon. An aldehyde differs from a ketone by having a hydrogen atom attached to the carbonyl group. In this case, methanal contains only one carbon atom, so it is not. Identify the longest continuous carbon chain that contains the formyl group. Methanal, as the simplest aldehyde compound, is crucial in establishing the principles of nomenclature for aldehydes and ketones.
from www.masterorganicchemistry.com
In this case, methanal contains only one carbon atom, so it is not. Methanal (formaldehyde, hcho) colourless, inflammable,. Where aldehydes and ketones differ. Methanal, as the simplest aldehyde compound, is crucial in establishing the principles of nomenclature for aldehydes and ketones. Identify the longest continuous carbon chain that contains the formyl group. The stem names of aldehydes and ketones are derived from those of the parent alkanes, defined by the longest continuous chain (lcc) of carbon. The stem names of aldehydes and ketones are derived from those of the parent alkanes, defined by the longest continuous chain (lcc) of carbon atoms that contains the functional group. Views 2,215,066 updated may 11 2018. An aldehyde differs from a ketone by having a hydrogen atom attached to the carbonyl group.
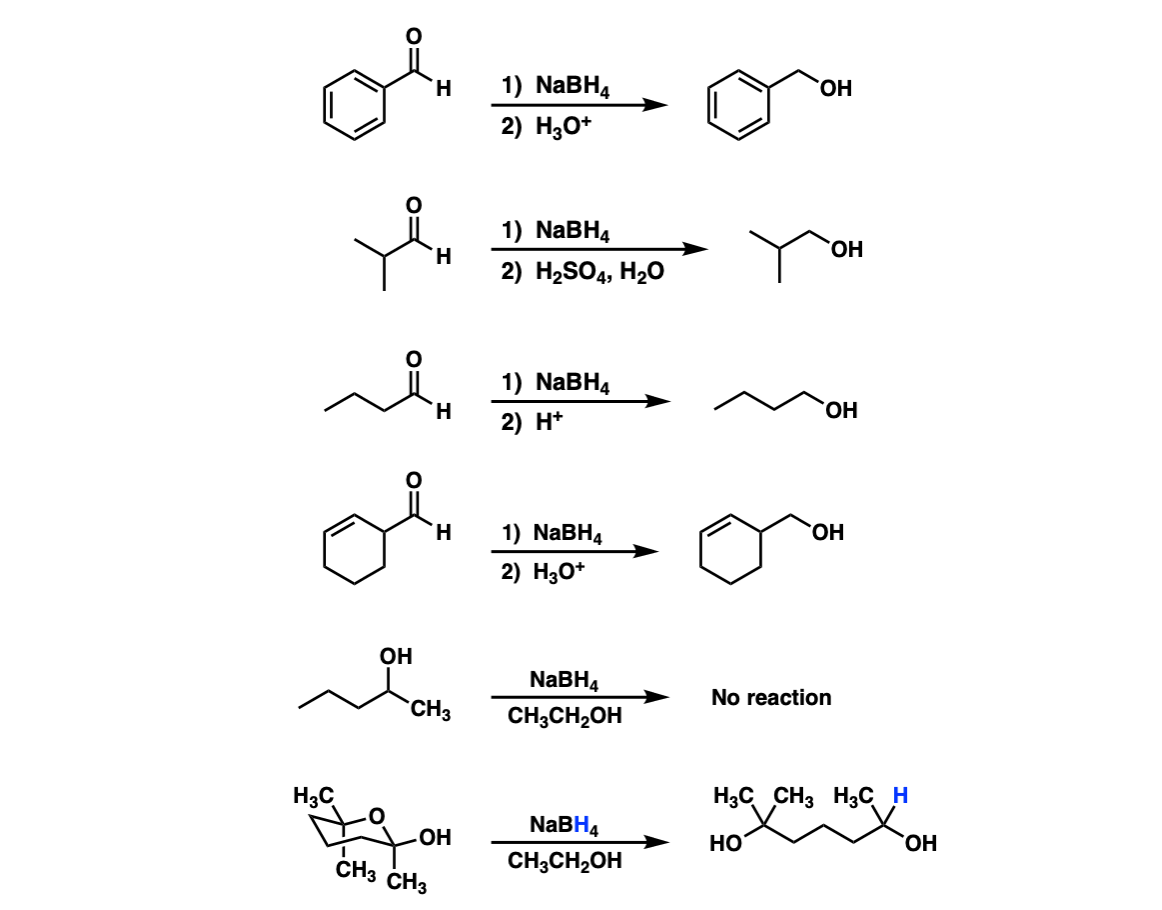
Addition of NaBH4 to aldehydes to give primary alcohols Master
Is Methanal An Aldehyde Where aldehydes and ketones differ. The stem names of aldehydes and ketones are derived from those of the parent alkanes, defined by the longest continuous chain (lcc) of carbon. Where aldehydes and ketones differ. Methanal, as the simplest aldehyde compound, is crucial in establishing the principles of nomenclature for aldehydes and ketones. In this case, methanal contains only one carbon atom, so it is not. An aldehyde differs from a ketone by having a hydrogen atom attached to the carbonyl group. The stem names of aldehydes and ketones are derived from those of the parent alkanes, defined by the longest continuous chain (lcc) of carbon atoms that contains the functional group. Identify the longest continuous carbon chain that contains the formyl group. Views 2,215,066 updated may 11 2018. Methanal (formaldehyde, hcho) colourless, inflammable,.
From www.animalia-life.club
Structural Formula Of Methanal Is Methanal An Aldehyde The stem names of aldehydes and ketones are derived from those of the parent alkanes, defined by the longest continuous chain (lcc) of carbon. An aldehyde differs from a ketone by having a hydrogen atom attached to the carbonyl group. Where aldehydes and ketones differ. Methanal, as the simplest aldehyde compound, is crucial in establishing the principles of nomenclature for. Is Methanal An Aldehyde.
From www.numerade.com
SOLVED The IUPAC name of the simplest aldehyde is Select one a Is Methanal An Aldehyde The stem names of aldehydes and ketones are derived from those of the parent alkanes, defined by the longest continuous chain (lcc) of carbon atoms that contains the functional group. Where aldehydes and ketones differ. Methanal, as the simplest aldehyde compound, is crucial in establishing the principles of nomenclature for aldehydes and ketones. Views 2,215,066 updated may 11 2018. The. Is Methanal An Aldehyde.
From exoozitth.blob.core.windows.net
Alpha Beta Hydroxy Aldehyde at Lisa Tucker blog Is Methanal An Aldehyde Where aldehydes and ketones differ. The stem names of aldehydes and ketones are derived from those of the parent alkanes, defined by the longest continuous chain (lcc) of carbon. Methanal, as the simplest aldehyde compound, is crucial in establishing the principles of nomenclature for aldehydes and ketones. Identify the longest continuous carbon chain that contains the formyl group. An aldehyde. Is Methanal An Aldehyde.
From byjus.com
Write the general formula for aldehyde. Is Methanal An Aldehyde The stem names of aldehydes and ketones are derived from those of the parent alkanes, defined by the longest continuous chain (lcc) of carbon atoms that contains the functional group. Methanal (formaldehyde, hcho) colourless, inflammable,. In this case, methanal contains only one carbon atom, so it is not. The stem names of aldehydes and ketones are derived from those of. Is Methanal An Aldehyde.
From study.com
Aldehyde Formula, Structure & Formation Lesson Is Methanal An Aldehyde Methanal, as the simplest aldehyde compound, is crucial in establishing the principles of nomenclature for aldehydes and ketones. The stem names of aldehydes and ketones are derived from those of the parent alkanes, defined by the longest continuous chain (lcc) of carbon. The stem names of aldehydes and ketones are derived from those of the parent alkanes, defined by the. Is Methanal An Aldehyde.
From www.animalia-life.club
Structural Formula Of Methanal Is Methanal An Aldehyde The stem names of aldehydes and ketones are derived from those of the parent alkanes, defined by the longest continuous chain (lcc) of carbon atoms that contains the functional group. The stem names of aldehydes and ketones are derived from those of the parent alkanes, defined by the longest continuous chain (lcc) of carbon. Identify the longest continuous carbon chain. Is Methanal An Aldehyde.
From ar.inspiredpencil.com
Structural Formula Of Methanal Is Methanal An Aldehyde Methanal, as the simplest aldehyde compound, is crucial in establishing the principles of nomenclature for aldehydes and ketones. Where aldehydes and ketones differ. The stem names of aldehydes and ketones are derived from those of the parent alkanes, defined by the longest continuous chain (lcc) of carbon. In this case, methanal contains only one carbon atom, so it is not.. Is Methanal An Aldehyde.
From www.numerade.com
SOLVED What is the IUPAC name for this compound? CH3 CHO 1ethanone B Is Methanal An Aldehyde Views 2,215,066 updated may 11 2018. Where aldehydes and ketones differ. Methanal (formaldehyde, hcho) colourless, inflammable,. Identify the longest continuous carbon chain that contains the formyl group. Methanal, as the simplest aldehyde compound, is crucial in establishing the principles of nomenclature for aldehydes and ketones. The stem names of aldehydes and ketones are derived from those of the parent alkanes,. Is Methanal An Aldehyde.
From byjus.com
When formaldehyde is treated with methanol in presence of dry HCI gas Is Methanal An Aldehyde The stem names of aldehydes and ketones are derived from those of the parent alkanes, defined by the longest continuous chain (lcc) of carbon atoms that contains the functional group. Identify the longest continuous carbon chain that contains the formyl group. Methanal (formaldehyde, hcho) colourless, inflammable,. Methanal, as the simplest aldehyde compound, is crucial in establishing the principles of nomenclature. Is Methanal An Aldehyde.
From www.numerade.com
SOLVED What is the IUPAC name for this compound? CH3CH ethanal 1 Is Methanal An Aldehyde Methanal, as the simplest aldehyde compound, is crucial in establishing the principles of nomenclature for aldehydes and ketones. In this case, methanal contains only one carbon atom, so it is not. Identify the longest continuous carbon chain that contains the formyl group. The stem names of aldehydes and ketones are derived from those of the parent alkanes, defined by the. Is Methanal An Aldehyde.
From learningchemistryeasily.blogspot.com
Organic Chemistry, Form, Part 14 Aldehydes Structure and Is Methanal An Aldehyde The stem names of aldehydes and ketones are derived from those of the parent alkanes, defined by the longest continuous chain (lcc) of carbon atoms that contains the functional group. Where aldehydes and ketones differ. Identify the longest continuous carbon chain that contains the formyl group. An aldehyde differs from a ketone by having a hydrogen atom attached to the. Is Methanal An Aldehyde.
From chem.libretexts.org
10.6.2. Strong Oxidizing Agents Chemistry LibreTexts Is Methanal An Aldehyde The stem names of aldehydes and ketones are derived from those of the parent alkanes, defined by the longest continuous chain (lcc) of carbon. An aldehyde differs from a ketone by having a hydrogen atom attached to the carbonyl group. In this case, methanal contains only one carbon atom, so it is not. Methanal (formaldehyde, hcho) colourless, inflammable,. Views 2,215,066. Is Methanal An Aldehyde.
From www.bartleby.com
The structural formula of hemiacetal that is formed when acetaldehyde Is Methanal An Aldehyde Identify the longest continuous carbon chain that contains the formyl group. The stem names of aldehydes and ketones are derived from those of the parent alkanes, defined by the longest continuous chain (lcc) of carbon atoms that contains the functional group. Methanal (formaldehyde, hcho) colourless, inflammable,. Where aldehydes and ketones differ. The stem names of aldehydes and ketones are derived. Is Methanal An Aldehyde.
From www.vedantu.com
Reduction of Aldehydes and Ketones for NEET Exam Is Methanal An Aldehyde Methanal, as the simplest aldehyde compound, is crucial in establishing the principles of nomenclature for aldehydes and ketones. Views 2,215,066 updated may 11 2018. Methanal (formaldehyde, hcho) colourless, inflammable,. Identify the longest continuous carbon chain that contains the formyl group. The stem names of aldehydes and ketones are derived from those of the parent alkanes, defined by the longest continuous. Is Methanal An Aldehyde.
From www.masterorganicchemistry.com
Addition of NaBH4 to aldehydes to give primary alcohols Master Is Methanal An Aldehyde In this case, methanal contains only one carbon atom, so it is not. An aldehyde differs from a ketone by having a hydrogen atom attached to the carbonyl group. Methanal, as the simplest aldehyde compound, is crucial in establishing the principles of nomenclature for aldehydes and ketones. Where aldehydes and ketones differ. The stem names of aldehydes and ketones are. Is Methanal An Aldehyde.
From www.reagent.co.uk
What is Aldehyde? The Science Blog Is Methanal An Aldehyde Views 2,215,066 updated may 11 2018. Methanal (formaldehyde, hcho) colourless, inflammable,. The stem names of aldehydes and ketones are derived from those of the parent alkanes, defined by the longest continuous chain (lcc) of carbon atoms that contains the functional group. Where aldehydes and ketones differ. In this case, methanal contains only one carbon atom, so it is not. Methanal,. Is Methanal An Aldehyde.
From www.numerade.com
SOLVED Classify each molecule as an aldehyde, ketone, or neither. Drag Is Methanal An Aldehyde Identify the longest continuous carbon chain that contains the formyl group. Where aldehydes and ketones differ. Views 2,215,066 updated may 11 2018. An aldehyde differs from a ketone by having a hydrogen atom attached to the carbonyl group. Methanal, as the simplest aldehyde compound, is crucial in establishing the principles of nomenclature for aldehydes and ketones. The stem names of. Is Methanal An Aldehyde.
From www.animalia-life.club
Structural Formula Of Methanal Is Methanal An Aldehyde Identify the longest continuous carbon chain that contains the formyl group. The stem names of aldehydes and ketones are derived from those of the parent alkanes, defined by the longest continuous chain (lcc) of carbon. In this case, methanal contains only one carbon atom, so it is not. The stem names of aldehydes and ketones are derived from those of. Is Methanal An Aldehyde.
From www.slideserve.com
PPT Aldehyde and Ketone Reactions PowerPoint Presentation ID142637 Is Methanal An Aldehyde In this case, methanal contains only one carbon atom, so it is not. An aldehyde differs from a ketone by having a hydrogen atom attached to the carbonyl group. Where aldehydes and ketones differ. Methanal, as the simplest aldehyde compound, is crucial in establishing the principles of nomenclature for aldehydes and ketones. The stem names of aldehydes and ketones are. Is Methanal An Aldehyde.
From www.slideserve.com
PPT Functional group PowerPoint Presentation, free download ID3068551 Is Methanal An Aldehyde Methanal, as the simplest aldehyde compound, is crucial in establishing the principles of nomenclature for aldehydes and ketones. In this case, methanal contains only one carbon atom, so it is not. The stem names of aldehydes and ketones are derived from those of the parent alkanes, defined by the longest continuous chain (lcc) of carbon. Where aldehydes and ketones differ.. Is Methanal An Aldehyde.
From courses.lumenlearning.com
14.10 Properties of Aldehydes and Ketones The Basics of General Is Methanal An Aldehyde Methanal (formaldehyde, hcho) colourless, inflammable,. An aldehyde differs from a ketone by having a hydrogen atom attached to the carbonyl group. Where aldehydes and ketones differ. Views 2,215,066 updated may 11 2018. Identify the longest continuous carbon chain that contains the formyl group. In this case, methanal contains only one carbon atom, so it is not. The stem names of. Is Methanal An Aldehyde.
From slideplayer.com
Aldehydes and ketones ppt download Is Methanal An Aldehyde Methanal, as the simplest aldehyde compound, is crucial in establishing the principles of nomenclature for aldehydes and ketones. Methanal (formaldehyde, hcho) colourless, inflammable,. An aldehyde differs from a ketone by having a hydrogen atom attached to the carbonyl group. The stem names of aldehydes and ketones are derived from those of the parent alkanes, defined by the longest continuous chain. Is Methanal An Aldehyde.
From www.masterorganicchemistry.com
Aldehydes and Ketones 14 Reactions With The Same Mechanism Is Methanal An Aldehyde The stem names of aldehydes and ketones are derived from those of the parent alkanes, defined by the longest continuous chain (lcc) of carbon atoms that contains the functional group. Views 2,215,066 updated may 11 2018. Methanal, as the simplest aldehyde compound, is crucial in establishing the principles of nomenclature for aldehydes and ketones. An aldehyde differs from a ketone. Is Methanal An Aldehyde.
From www.slideserve.com
PPT Topic Functional Group 5Aldehydes PowerPoint Presentation Is Methanal An Aldehyde In this case, methanal contains only one carbon atom, so it is not. Identify the longest continuous carbon chain that contains the formyl group. An aldehyde differs from a ketone by having a hydrogen atom attached to the carbonyl group. The stem names of aldehydes and ketones are derived from those of the parent alkanes, defined by the longest continuous. Is Methanal An Aldehyde.
From byjus.com
Formaldehyde Formula Chemical and Structural Formula of Methanal Is Methanal An Aldehyde Methanal (formaldehyde, hcho) colourless, inflammable,. Where aldehydes and ketones differ. Identify the longest continuous carbon chain that contains the formyl group. In this case, methanal contains only one carbon atom, so it is not. An aldehyde differs from a ketone by having a hydrogen atom attached to the carbonyl group. Views 2,215,066 updated may 11 2018. Methanal, as the simplest. Is Methanal An Aldehyde.
From www.passmyexams.co.uk
Oxidation of Methanol Easy exam revision notes for GSCE Chemistry Is Methanal An Aldehyde The stem names of aldehydes and ketones are derived from those of the parent alkanes, defined by the longest continuous chain (lcc) of carbon. Methanal (formaldehyde, hcho) colourless, inflammable,. An aldehyde differs from a ketone by having a hydrogen atom attached to the carbonyl group. The stem names of aldehydes and ketones are derived from those of the parent alkanes,. Is Methanal An Aldehyde.
From www.vedantu.com
How is ethanol prepared by methanal by using Grignard reagent? Is Methanal An Aldehyde Where aldehydes and ketones differ. An aldehyde differs from a ketone by having a hydrogen atom attached to the carbonyl group. The stem names of aldehydes and ketones are derived from those of the parent alkanes, defined by the longest continuous chain (lcc) of carbon. Methanal, as the simplest aldehyde compound, is crucial in establishing the principles of nomenclature for. Is Methanal An Aldehyde.
From www.numerade.com
SOLVED As methanol is converted to methanal, and then methanoic acid Is Methanal An Aldehyde Methanal, as the simplest aldehyde compound, is crucial in establishing the principles of nomenclature for aldehydes and ketones. Methanal (formaldehyde, hcho) colourless, inflammable,. An aldehyde differs from a ketone by having a hydrogen atom attached to the carbonyl group. Where aldehydes and ketones differ. The stem names of aldehydes and ketones are derived from those of the parent alkanes, defined. Is Methanal An Aldehyde.
From www.slideserve.com
PPT Chapter 19 PowerPoint Presentation, free download ID6662749 Is Methanal An Aldehyde The stem names of aldehydes and ketones are derived from those of the parent alkanes, defined by the longest continuous chain (lcc) of carbon. Identify the longest continuous carbon chain that contains the formyl group. Methanal, as the simplest aldehyde compound, is crucial in establishing the principles of nomenclature for aldehydes and ketones. Methanal (formaldehyde, hcho) colourless, inflammable,. Where aldehydes. Is Methanal An Aldehyde.
From askfilo.com
Thus, its IUPAC name is methanal. IUPAC name of acetaldehyde is Filo Is Methanal An Aldehyde The stem names of aldehydes and ketones are derived from those of the parent alkanes, defined by the longest continuous chain (lcc) of carbon atoms that contains the functional group. In this case, methanal contains only one carbon atom, so it is not. Methanal (formaldehyde, hcho) colourless, inflammable,. Methanal, as the simplest aldehyde compound, is crucial in establishing the principles. Is Methanal An Aldehyde.
From www.alamy.com
Formaldehyde High Resolution Stock Photography and Images Alamy Is Methanal An Aldehyde Where aldehydes and ketones differ. Views 2,215,066 updated may 11 2018. Methanal (formaldehyde, hcho) colourless, inflammable,. Identify the longest continuous carbon chain that contains the formyl group. In this case, methanal contains only one carbon atom, so it is not. The stem names of aldehydes and ketones are derived from those of the parent alkanes, defined by the longest continuous. Is Methanal An Aldehyde.
From ar.inspiredpencil.com
Acetaldehyde Reaction Is Methanal An Aldehyde An aldehyde differs from a ketone by having a hydrogen atom attached to the carbonyl group. Where aldehydes and ketones differ. Identify the longest continuous carbon chain that contains the formyl group. Views 2,215,066 updated may 11 2018. Methanal, as the simplest aldehyde compound, is crucial in establishing the principles of nomenclature for aldehydes and ketones. The stem names of. Is Methanal An Aldehyde.
From www.masterorganicchemistry.com
Aldehydes and Ketones 14 Reactions With The Same Mechanism Is Methanal An Aldehyde The stem names of aldehydes and ketones are derived from those of the parent alkanes, defined by the longest continuous chain (lcc) of carbon. Where aldehydes and ketones differ. The stem names of aldehydes and ketones are derived from those of the parent alkanes, defined by the longest continuous chain (lcc) of carbon atoms that contains the functional group. Identify. Is Methanal An Aldehyde.
From slideplayer.com
Organic Chemistry (Functional Groups) ppt download Is Methanal An Aldehyde Methanal, as the simplest aldehyde compound, is crucial in establishing the principles of nomenclature for aldehydes and ketones. Where aldehydes and ketones differ. The stem names of aldehydes and ketones are derived from those of the parent alkanes, defined by the longest continuous chain (lcc) of carbon. Identify the longest continuous carbon chain that contains the formyl group. An aldehyde. Is Methanal An Aldehyde.
From www.vectorstock.com
Formaldehyde methanal molecule important indoor Vector Image Is Methanal An Aldehyde An aldehyde differs from a ketone by having a hydrogen atom attached to the carbonyl group. Where aldehydes and ketones differ. Methanal (formaldehyde, hcho) colourless, inflammable,. Views 2,215,066 updated may 11 2018. The stem names of aldehydes and ketones are derived from those of the parent alkanes, defined by the longest continuous chain (lcc) of carbon atoms that contains the. Is Methanal An Aldehyde.