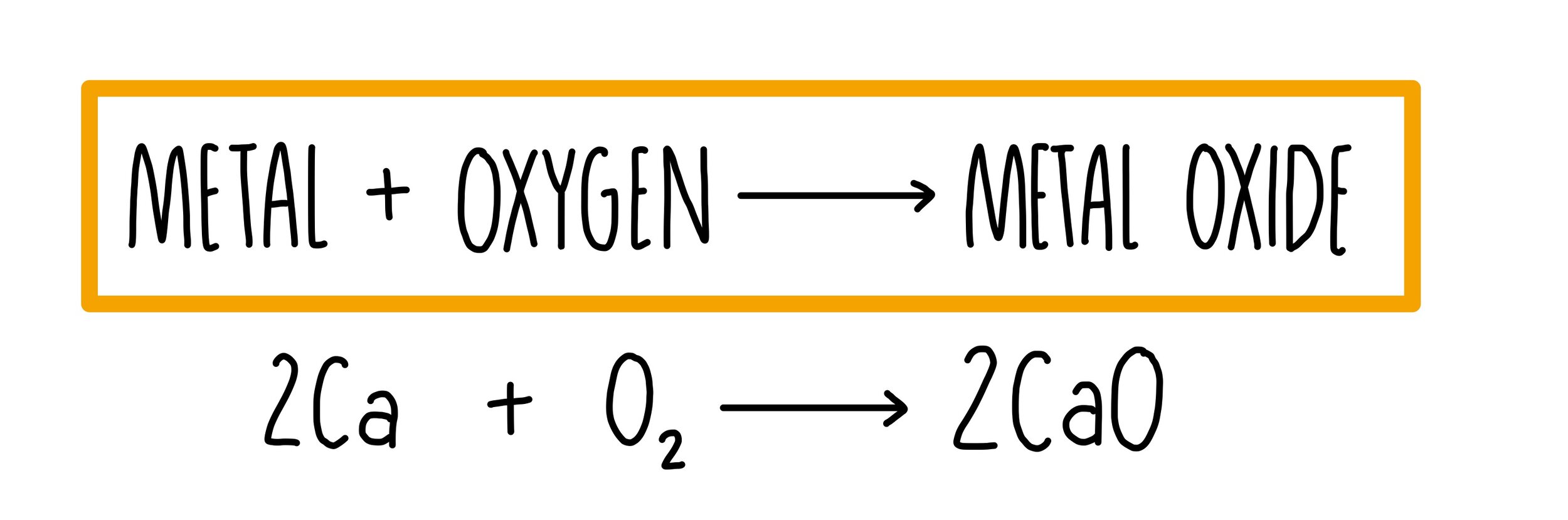Metal + Oxygen Example . What happens when a metal reacts with oxygen? Metals react with oxygen to produce metal oxide. What is the product called? Metal + oxygen reactions chemistry tutorial key concepts. With metals, oxygen forms oxides that are largely ionic in character. All metals react with oxygen except silver (ag (s)), platinum (pt (s)) and gold (au (s)). Watch this video for a. There are general trends in the reactions between main group elements and oxygen: The speed of the reaction depends on the reactivity of the metal. Many metals react with oxygen to form metal oxides. Potassium and sodium are soft metal which are easily cut exposing a shiny surface which changes to dull rapidly. Metals, on being burnt in air, react with the oxygen to form metal oxides. Most nonmetals form the oxide with. The reaction of metals with air (oxygen). When metals react with oxygen, metal oxides are formed.
from www.thesciencehive.co.uk
The reaction of metals with air (oxygen). Metals react with oxygen to produce metal oxide. Watch this video for a. Metal + oxygen reactions chemistry tutorial key concepts. What is the product called? Potassium, sodium, lithium, calcium and magnesium react with oxygen and burn in air. Why is phosphorus not kept in open? Many metals react with oxygen to form metal oxides. When metals react with oxygen, metal oxides are formed. Metals, on being burnt in air, react with the oxygen to form metal oxides.
Group 2* — the science sauce
Metal + Oxygen Example The change from shiny to. Phosphorus is a very reactive non metal.if it is kept in open air it will react with oxygen and catch. What happens when a metal reacts with oxygen? Most nonmetals form the oxide with. Watch this video for a. Metal + oxygen reactions chemistry tutorial key concepts. Metals, on being burnt in air, react with the oxygen to form metal oxides. How can we represent the general reaction between a metal and oxygen?. What is the product called? There are general trends in the reactions between main group elements and oxygen: Many metals react with oxygen to form metal oxides. The reaction of metals with air (oxygen). Metals react with oxygen to produce metal oxide. The speed of the reaction depends on the reactivity of the metal. Why is phosphorus not kept in open? When metals react with oxygen, metal oxides are formed.
From www.slideserve.com
PPT Chemistry Year 10 Chemical reactions PowerPoint Presentation Metal + Oxygen Example What happens when a metal reacts with oxygen? The reaction of metals with air (oxygen). What is the product called? All metals react with oxygen except silver (ag (s)), platinum (pt (s)) and gold (au (s)). Metals react with oxygen to produce metal oxide. Many metals react with oxygen to form metal oxides. When metals react with oxygen, metal oxides. Metal + Oxygen Example.
From www.youtube.com
Reactivity of Metal with oxygen(Chemical Properties)(Hindi) YouTube Metal + Oxygen Example All metals react with oxygen except silver (ag (s)), platinum (pt (s)) and gold (au (s)). Most nonmetals form the oxide with. The change from shiny to. With metals, oxygen forms oxides that are largely ionic in character. Why is phosphorus not kept in open? Potassium, sodium, lithium, calcium and magnesium react with oxygen and burn in air. What happens. Metal + Oxygen Example.
From www.slideserve.com
PPT Reactions of Metals PowerPoint Presentation, free download ID Metal + Oxygen Example The reaction of metals with air (oxygen). The change from shiny to. Potassium and sodium are soft metal which are easily cut exposing a shiny surface which changes to dull rapidly. Phosphorus is a very reactive non metal.if it is kept in open air it will react with oxygen and catch. What happens when a metal reacts with oxygen? How. Metal + Oxygen Example.
From www.youtube.com
Standard Reactions Metal + Oxygen YouTube Metal + Oxygen Example Metal + oxygen reactions chemistry tutorial key concepts. Many metals react with oxygen to form metal oxides. When metals react with oxygen, metal oxides are formed. Potassium and sodium are soft metal which are easily cut exposing a shiny surface which changes to dull rapidly. The change from shiny to. There are general trends in the reactions between main group. Metal + Oxygen Example.
From www.youtube.com
Reactions of Oxygen with Metals, Chemistry Lecture Sabaq.pk YouTube Metal + Oxygen Example Phosphorus is a very reactive non metal.if it is kept in open air it will react with oxygen and catch. Why is phosphorus not kept in open? The speed of the reaction depends on the reactivity of the metal. The reaction of metals with air (oxygen). Potassium and sodium are soft metal which are easily cut exposing a shiny surface. Metal + Oxygen Example.
From www.slideserve.com
PPT Unit 8 Chemical Reactions PowerPoint Presentation, free download Metal + Oxygen Example Phosphorus is a very reactive non metal.if it is kept in open air it will react with oxygen and catch. The speed of the reaction depends on the reactivity of the metal. With metals, oxygen forms oxides that are largely ionic in character. How can we represent the general reaction between a metal and oxygen?. Metals react with oxygen to. Metal + Oxygen Example.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID Metal + Oxygen Example With metals, oxygen forms oxides that are largely ionic in character. When metals react with oxygen, metal oxides are formed. Phosphorus is a very reactive non metal.if it is kept in open air it will react with oxygen and catch. Why is phosphorus not kept in open? How can we represent the general reaction between a metal and oxygen?. What. Metal + Oxygen Example.
From www.teachoo.com
The Chemical Reaction Involved in the Corrosion of Iron Metal Metal + Oxygen Example Why is phosphorus not kept in open? Potassium and sodium are soft metal which are easily cut exposing a shiny surface which changes to dull rapidly. When metals react with oxygen, metal oxides are formed. The reaction of metals with air (oxygen). How can we represent the general reaction between a metal and oxygen?. Potassium, sodium, lithium, calcium and magnesium. Metal + Oxygen Example.
From www.teachoo.com
Reaction of Metals and Nonmetals with Oxygen Concepts Metal + Oxygen Example Watch this video for a. All metals react with oxygen except silver (ag (s)), platinum (pt (s)) and gold (au (s)). How can we represent the general reaction between a metal and oxygen?. Potassium and sodium are soft metal which are easily cut exposing a shiny surface which changes to dull rapidly. Many metals react with oxygen to form metal. Metal + Oxygen Example.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 2.1.1 Group 1 (Alkali Metals)翰林国际教育 Metal + Oxygen Example Potassium, sodium, lithium, calcium and magnesium react with oxygen and burn in air. All metals react with oxygen except silver (ag (s)), platinum (pt (s)) and gold (au (s)). What happens when a metal reacts with oxygen? The change from shiny to. Watch this video for a. When metals react with oxygen, metal oxides are formed. The speed of the. Metal + Oxygen Example.
From www.slideserve.com
PPT Reacting metals with oxygen PowerPoint Presentation ID6645971 Metal + Oxygen Example Most nonmetals form the oxide with. Phosphorus is a very reactive non metal.if it is kept in open air it will react with oxygen and catch. The change from shiny to. Watch this video for a. The speed of the reaction depends on the reactivity of the metal. Metals react with oxygen to produce metal oxide. There are general trends. Metal + Oxygen Example.
From www.slideserve.com
PPT Reacting metals with oxygen PowerPoint Presentation, free Metal + Oxygen Example The speed of the reaction depends on the reactivity of the metal. How can we represent the general reaction between a metal and oxygen?. Watch this video for a. The reaction of metals with air (oxygen). Phosphorus is a very reactive non metal.if it is kept in open air it will react with oxygen and catch. Potassium and sodium are. Metal + Oxygen Example.
From sites.google.com
Oxygen Metal + Oxygen Example Phosphorus is a very reactive non metal.if it is kept in open air it will react with oxygen and catch. All metals react with oxygen except silver (ag (s)), platinum (pt (s)) and gold (au (s)). Metals, on being burnt in air, react with the oxygen to form metal oxides. The speed of the reaction depends on the reactivity of. Metal + Oxygen Example.
From ellaaressims.blogspot.com
Potassium Reaction With Oxygen EllaaresSims Metal + Oxygen Example What is the product called? Metals, on being burnt in air, react with the oxygen to form metal oxides. All metals react with oxygen except silver (ag (s)), platinum (pt (s)) and gold (au (s)). Many metals react with oxygen to form metal oxides. The change from shiny to. Metals react with oxygen to produce metal oxide. Most nonmetals form. Metal + Oxygen Example.
From www.slideshare.net
Reaction of metal with oxygen Metal + Oxygen Example Most nonmetals form the oxide with. Metal + oxygen reactions chemistry tutorial key concepts. The reaction of metals with air (oxygen). What happens when a metal reacts with oxygen? When metals react with oxygen, metal oxides are formed. Potassium, sodium, lithium, calcium and magnesium react with oxygen and burn in air. With metals, oxygen forms oxides that are largely ionic. Metal + Oxygen Example.
From www.teachoo.com
Reaction of Metals and Nonmetals with Oxygen Concepts Metal + Oxygen Example Potassium, sodium, lithium, calcium and magnesium react with oxygen and burn in air. With metals, oxygen forms oxides that are largely ionic in character. Most nonmetals form the oxide with. When metals react with oxygen, metal oxides are formed. Why is phosphorus not kept in open? Metals, on being burnt in air, react with the oxygen to form metal oxides.. Metal + Oxygen Example.
From www.teachoo.com
Reaction of Metals and Nonmetals with Oxygen Concepts Metal + Oxygen Example The speed of the reaction depends on the reactivity of the metal. How can we represent the general reaction between a metal and oxygen?. When metals react with oxygen, metal oxides are formed. There are general trends in the reactions between main group elements and oxygen: The change from shiny to. All metals react with oxygen except silver (ag (s)),. Metal + Oxygen Example.
From www.youtube.com
Reaction of Metals with Oxygen Chemistry YouTube Metal + Oxygen Example Metals, on being burnt in air, react with the oxygen to form metal oxides. What happens when a metal reacts with oxygen? When metals react with oxygen, metal oxides are formed. Potassium and sodium are soft metal which are easily cut exposing a shiny surface which changes to dull rapidly. How can we represent the general reaction between a metal. Metal + Oxygen Example.
From spmchemistry.blog.onlinetuition.com.my
Group 1 Elements Alkali Metals SPM Chemistry Metal + Oxygen Example Most nonmetals form the oxide with. The reaction of metals with air (oxygen). Watch this video for a. When metals react with oxygen, metal oxides are formed. Metals, on being burnt in air, react with the oxygen to form metal oxides. Metals react with oxygen to produce metal oxide. The change from shiny to. Potassium, sodium, lithium, calcium and magnesium. Metal + Oxygen Example.
From www.youtube.com
MELC BASED LESSON SCIENCE 5 Changes in Matter in the Presence or Metal + Oxygen Example When metals react with oxygen, metal oxides are formed. The reaction of metals with air (oxygen). How can we represent the general reaction between a metal and oxygen?. Metals react with oxygen to produce metal oxide. Most nonmetals form the oxide with. What is the product called? All metals react with oxygen except silver (ag (s)), platinum (pt (s)) and. Metal + Oxygen Example.
From www.slideserve.com
PPT Reactions of Metals PowerPoint Presentation, free download ID Metal + Oxygen Example There are general trends in the reactions between main group elements and oxygen: What happens when a metal reacts with oxygen? What is the product called? Phosphorus is a very reactive non metal.if it is kept in open air it will react with oxygen and catch. Most nonmetals form the oxide with. Potassium and sodium are soft metal which are. Metal + Oxygen Example.
From www.onlinemathlearning.com
Reaction of Metals (examples, answers, activities, experiment, videos) Metal + Oxygen Example The speed of the reaction depends on the reactivity of the metal. The change from shiny to. How can we represent the general reaction between a metal and oxygen?. Potassium and sodium are soft metal which are easily cut exposing a shiny surface which changes to dull rapidly. Many metals react with oxygen to form metal oxides. Metals react with. Metal + Oxygen Example.
From www.youtube.com
AE6 Non metal oxides YouTube Metal + Oxygen Example Metals react with oxygen to produce metal oxide. Phosphorus is a very reactive non metal.if it is kept in open air it will react with oxygen and catch. The change from shiny to. Why is phosphorus not kept in open? Many metals react with oxygen to form metal oxides. All metals react with oxygen except silver (ag (s)), platinum (pt. Metal + Oxygen Example.
From www.embibe.com
What is Oxygen? Definition, Properties, Elements, Uses Metal + Oxygen Example Why is phosphorus not kept in open? What happens when a metal reacts with oxygen? Potassium, sodium, lithium, calcium and magnesium react with oxygen and burn in air. What is the product called? There are general trends in the reactions between main group elements and oxygen: The speed of the reaction depends on the reactivity of the metal. Metal +. Metal + Oxygen Example.
From www.bartleby.com
Metals and Nonmetals bartleby Metal + Oxygen Example When metals react with oxygen, metal oxides are formed. Potassium, sodium, lithium, calcium and magnesium react with oxygen and burn in air. All metals react with oxygen except silver (ag (s)), platinum (pt (s)) and gold (au (s)). Metals react with oxygen to produce metal oxide. Why is phosphorus not kept in open? The speed of the reaction depends on. Metal + Oxygen Example.
From www.teachoo.com
Reaction of Metals and Nonmetals with Oxygen Concepts Metal + Oxygen Example Metal + oxygen reactions chemistry tutorial key concepts. How can we represent the general reaction between a metal and oxygen?. Metals react with oxygen to produce metal oxide. With metals, oxygen forms oxides that are largely ionic in character. What happens when a metal reacts with oxygen? Potassium and sodium are soft metal which are easily cut exposing a shiny. Metal + Oxygen Example.
From www.youtube.com
Reactions of Oxygen with Nonmetals, Chemistry Lecture Sabaq.pk YouTube Metal + Oxygen Example Many metals react with oxygen to form metal oxides. Metals, on being burnt in air, react with the oxygen to form metal oxides. Metal + oxygen reactions chemistry tutorial key concepts. Most nonmetals form the oxide with. The reaction of metals with air (oxygen). How can we represent the general reaction between a metal and oxygen?. Watch this video for. Metal + Oxygen Example.
From www.thesciencehive.co.uk
Group 2* — the science sauce Metal + Oxygen Example All metals react with oxygen except silver (ag (s)), platinum (pt (s)) and gold (au (s)). Phosphorus is a very reactive non metal.if it is kept in open air it will react with oxygen and catch. Potassium and sodium are soft metal which are easily cut exposing a shiny surface which changes to dull rapidly. Potassium, sodium, lithium, calcium and. Metal + Oxygen Example.
From www.teachoo.com
Chemical Properties of Metals [with Reaction Examples] Teachoo Metal + Oxygen Example Watch this video for a. When metals react with oxygen, metal oxides are formed. Most nonmetals form the oxide with. Potassium, sodium, lithium, calcium and magnesium react with oxygen and burn in air. The speed of the reaction depends on the reactivity of the metal. Phosphorus is a very reactive non metal.if it is kept in open air it will. Metal + Oxygen Example.
From analiticaderetail.com
folyosó Hirdetés karmester metal oxide oxygen tanú Bájos fórum Metal + Oxygen Example Metal + oxygen reactions chemistry tutorial key concepts. The change from shiny to. What is the product called? Metals react with oxygen to produce metal oxide. Phosphorus is a very reactive non metal.if it is kept in open air it will react with oxygen and catch. How can we represent the general reaction between a metal and oxygen?. With metals,. Metal + Oxygen Example.
From www.slideserve.com
PPT CHAPTER 3 METALS AND NON METALS PowerPoint Presentation, free Metal + Oxygen Example There are general trends in the reactions between main group elements and oxygen: The reaction of metals with air (oxygen). Potassium, sodium, lithium, calcium and magnesium react with oxygen and burn in air. Phosphorus is a very reactive non metal.if it is kept in open air it will react with oxygen and catch. Watch this video for a. Why is. Metal + Oxygen Example.
From godsgracefaces.us
Oxygen Please??? Redemption Metal + Oxygen Example Potassium and sodium are soft metal which are easily cut exposing a shiny surface which changes to dull rapidly. The speed of the reaction depends on the reactivity of the metal. All metals react with oxygen except silver (ag (s)), platinum (pt (s)) and gold (au (s)). Phosphorus is a very reactive non metal.if it is kept in open air. Metal + Oxygen Example.
From www.youtube.com
Reactions of nonmetal with oxygen Chemical properties of nonmetals Metal + Oxygen Example With metals, oxygen forms oxides that are largely ionic in character. Why is phosphorus not kept in open? Phosphorus is a very reactive non metal.if it is kept in open air it will react with oxygen and catch. What happens when a metal reacts with oxygen? Most nonmetals form the oxide with. Watch this video for a. Potassium and sodium. Metal + Oxygen Example.
From www.youtube.com
The Reaction of Metals with Oxygen YouTube Metal + Oxygen Example The change from shiny to. How can we represent the general reaction between a metal and oxygen?. Watch this video for a. When metals react with oxygen, metal oxides are formed. What is the product called? Potassium, sodium, lithium, calcium and magnesium react with oxygen and burn in air. All metals react with oxygen except silver (ag (s)), platinum (pt. Metal + Oxygen Example.
From slideplayer.com
1. ppt download Metal + Oxygen Example Metals, on being burnt in air, react with the oxygen to form metal oxides. Why is phosphorus not kept in open? Metals react with oxygen to produce metal oxide. Many metals react with oxygen to form metal oxides. With metals, oxygen forms oxides that are largely ionic in character. Most nonmetals form the oxide with. Phosphorus is a very reactive. Metal + Oxygen Example.