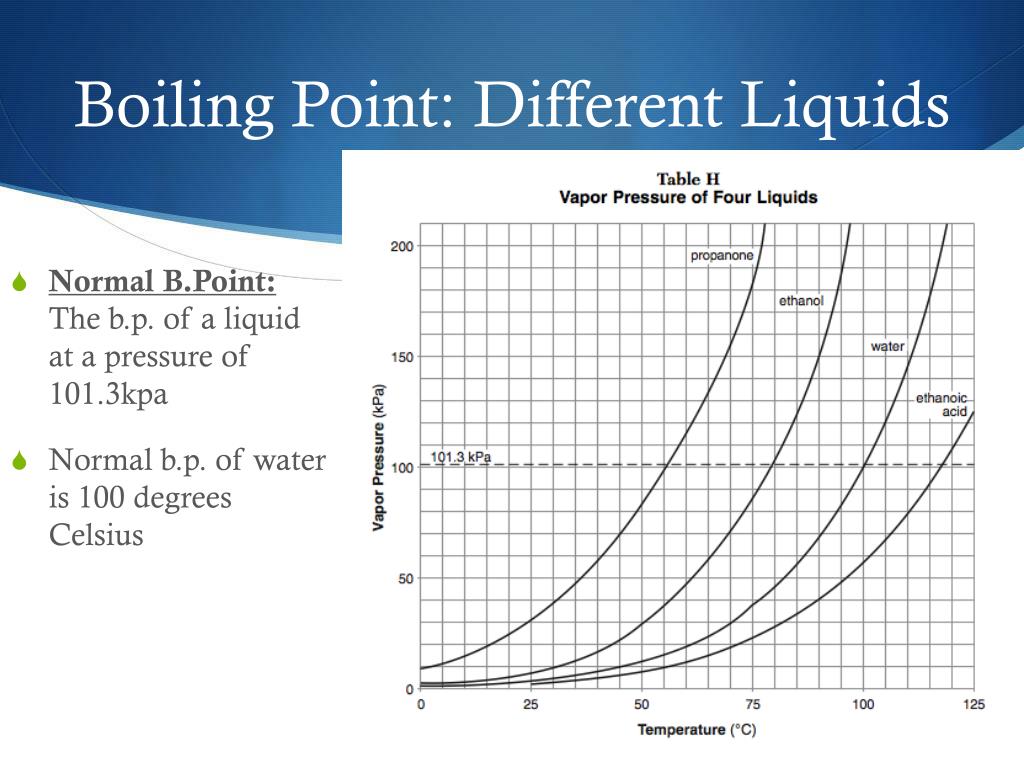
What Is Water S Highest Boiling Point . Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. However, in this section we learn that the boiling point of a liquid depends on pressure. The boiling point for water is 212 ºf or 100 ºc, whereas the boiling point of salt water is about 102 ºc. The boiling point of a liquid varies according to the applied pressure; The high boiling point of water, which is 100°c (212°f) at standard atmospheric pressure, is a result of several factors, including the. Impurities in water, like salt,. It is well known that boiling point depends on temperature. Most know that water boils at 100 degrees celsius. The normal boiling point is the temperature at which the vapour pressure is equal to.
from exyqxtcdu.blob.core.windows.net
Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. It is well known that boiling point depends on temperature. The boiling point of a liquid varies according to the applied pressure; The high boiling point of water, which is 100°c (212°f) at standard atmospheric pressure, is a result of several factors, including the. However, in this section we learn that the boiling point of a liquid depends on pressure. The boiling point for water is 212 ºf or 100 ºc, whereas the boiling point of salt water is about 102 ºc. The normal boiling point is the temperature at which the vapour pressure is equal to. Impurities in water, like salt,. Most know that water boils at 100 degrees celsius.
What Is The Boiling Point Constant Of Water at Beatrice Hughes blog
What Is Water S Highest Boiling Point Impurities in water, like salt,. Most know that water boils at 100 degrees celsius. It is well known that boiling point depends on temperature. The boiling point for water is 212 ºf or 100 ºc, whereas the boiling point of salt water is about 102 ºc. Impurities in water, like salt,. The high boiling point of water, which is 100°c (212°f) at standard atmospheric pressure, is a result of several factors, including the. The boiling point of a liquid varies according to the applied pressure; However, in this section we learn that the boiling point of a liquid depends on pressure. Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. The normal boiling point is the temperature at which the vapour pressure is equal to.
From www.dreamstime.com
Boiling Point of Water in Different Altitude Meter Levels Outline What Is Water S Highest Boiling Point It is well known that boiling point depends on temperature. Impurities in water, like salt,. The high boiling point of water, which is 100°c (212°f) at standard atmospheric pressure, is a result of several factors, including the. The normal boiling point is the temperature at which the vapour pressure is equal to. The boiling point for water is 212 ºf. What Is Water S Highest Boiling Point.
From www.slideserve.com
PPT Freezing/Melting and Boiling Points PowerPoint Presentation, free What Is Water S Highest Boiling Point The boiling point for water is 212 ºf or 100 ºc, whereas the boiling point of salt water is about 102 ºc. The normal boiling point is the temperature at which the vapour pressure is equal to. However, in this section we learn that the boiling point of a liquid depends on pressure. Water boils at a lower temperature as. What Is Water S Highest Boiling Point.
From exometidq.blob.core.windows.net
What Is Water S Boiling Point In Fahrenheit And Celsius at Adam Weitz blog What Is Water S Highest Boiling Point The high boiling point of water, which is 100°c (212°f) at standard atmospheric pressure, is a result of several factors, including the. The boiling point of a liquid varies according to the applied pressure; It is well known that boiling point depends on temperature. Impurities in water, like salt,. The normal boiling point is the temperature at which the vapour. What Is Water S Highest Boiling Point.
From howchimp.com
What Is the Boiling Point of Water in Kelvin, Celsius, and Fahrenheit What Is Water S Highest Boiling Point Impurities in water, like salt,. Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. The normal boiling point is the temperature at which the vapour pressure is equal to. The boiling point of a liquid varies according to the applied pressure; Most know that. What Is Water S Highest Boiling Point.
From www.worksheetsplanet.com
What is Boiling Point What Is Water S Highest Boiling Point The boiling point of a liquid varies according to the applied pressure; Impurities in water, like salt,. Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. However, in this section we learn that the boiling point of a liquid depends on pressure. The high. What Is Water S Highest Boiling Point.
From marshallkruwmathis.blogspot.com
What is the Boiling Point of Water MarshallkruwMathis What Is Water S Highest Boiling Point The boiling point for water is 212 ºf or 100 ºc, whereas the boiling point of salt water is about 102 ºc. The normal boiling point is the temperature at which the vapour pressure is equal to. Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if. What Is Water S Highest Boiling Point.
From dxoemuxwe.blob.core.windows.net
Does Boiling Water Heat A Room at Tarah Hedrick blog What Is Water S Highest Boiling Point The boiling point of a liquid varies according to the applied pressure; Impurities in water, like salt,. It is well known that boiling point depends on temperature. The high boiling point of water, which is 100°c (212°f) at standard atmospheric pressure, is a result of several factors, including the. The normal boiling point is the temperature at which the vapour. What Is Water S Highest Boiling Point.
From www.dreamstime.com
Boiling and Evaporation, Freezing and Melting Points of Water Stock What Is Water S Highest Boiling Point However, in this section we learn that the boiling point of a liquid depends on pressure. Most know that water boils at 100 degrees celsius. The boiling point for water is 212 ºf or 100 ºc, whereas the boiling point of salt water is about 102 ºc. Impurities in water, like salt,. Water boils at a lower temperature as you. What Is Water S Highest Boiling Point.
From brainly.com
This graph shows the melting and boiling points of the alkali metals What Is Water S Highest Boiling Point The normal boiling point is the temperature at which the vapour pressure is equal to. The high boiling point of water, which is 100°c (212°f) at standard atmospheric pressure, is a result of several factors, including the. However, in this section we learn that the boiling point of a liquid depends on pressure. The boiling point of a liquid varies. What Is Water S Highest Boiling Point.
From www.slideserve.com
PPT Opening Assignment PowerPoint Presentation, free download ID What Is Water S Highest Boiling Point Impurities in water, like salt,. However, in this section we learn that the boiling point of a liquid depends on pressure. The high boiling point of water, which is 100°c (212°f) at standard atmospheric pressure, is a result of several factors, including the. It is well known that boiling point depends on temperature. Most know that water boils at 100. What Is Water S Highest Boiling Point.
From joiaepnij.blob.core.windows.net
When The Water Is Boiling A at Ralph Hallett blog What Is Water S Highest Boiling Point The normal boiling point is the temperature at which the vapour pressure is equal to. The boiling point for water is 212 ºf or 100 ºc, whereas the boiling point of salt water is about 102 ºc. Impurities in water, like salt,. The high boiling point of water, which is 100°c (212°f) at standard atmospheric pressure, is a result of. What Is Water S Highest Boiling Point.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps What Is Water S Highest Boiling Point The boiling point of a liquid varies according to the applied pressure; The high boiling point of water, which is 100°c (212°f) at standard atmospheric pressure, is a result of several factors, including the. The boiling point for water is 212 ºf or 100 ºc, whereas the boiling point of salt water is about 102 ºc. Impurities in water, like. What Is Water S Highest Boiling Point.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps What Is Water S Highest Boiling Point It is well known that boiling point depends on temperature. The high boiling point of water, which is 100°c (212°f) at standard atmospheric pressure, is a result of several factors, including the. Impurities in water, like salt,. Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if. What Is Water S Highest Boiling Point.
From studiousguy.com
Boiling Point Examples in Everyday Life StudiousGuy What Is Water S Highest Boiling Point The boiling point of a liquid varies according to the applied pressure; The normal boiling point is the temperature at which the vapour pressure is equal to. It is well known that boiling point depends on temperature. The high boiling point of water, which is 100°c (212°f) at standard atmospheric pressure, is a result of several factors, including the. Water. What Is Water S Highest Boiling Point.
From www.thoughtco.com
What Is the Boiling Point of Water? What Is Water S Highest Boiling Point The boiling point of a liquid varies according to the applied pressure; Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. Impurities in water, like salt,. The high boiling point of water, which is 100°c (212°f) at standard atmospheric pressure, is a result of. What Is Water S Highest Boiling Point.
From jsmithmoore.com
Boiling point of ethanol celsius What Is Water S Highest Boiling Point Impurities in water, like salt,. The boiling point for water is 212 ºf or 100 ºc, whereas the boiling point of salt water is about 102 ºc. Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. However, in this section we learn that the. What Is Water S Highest Boiling Point.
From www.learnatnoon.com
The boiling point of water and alcohol explained Noon Academy What Is Water S Highest Boiling Point The boiling point for water is 212 ºf or 100 ºc, whereas the boiling point of salt water is about 102 ºc. Most know that water boils at 100 degrees celsius. Impurities in water, like salt,. The boiling point of a liquid varies according to the applied pressure; Water boils at a lower temperature as you gain altitude (e.g., going. What Is Water S Highest Boiling Point.
From blog.thermoworks.com
Thermal Secrets to Boiling Point Calibration ThermoWorks What Is Water S Highest Boiling Point The normal boiling point is the temperature at which the vapour pressure is equal to. The boiling point for water is 212 ºf or 100 ºc, whereas the boiling point of salt water is about 102 ºc. However, in this section we learn that the boiling point of a liquid depends on pressure. Water boils at a lower temperature as. What Is Water S Highest Boiling Point.
From www.slideserve.com
PPT Entry Task Nov 27 th Block 1 PowerPoint Presentation, free What Is Water S Highest Boiling Point Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. Impurities in water, like salt,. However, in this section we learn that the boiling point of a liquid depends on pressure. The boiling point for water is 212 ºf or 100 ºc, whereas the boiling. What Is Water S Highest Boiling Point.
From waterspecialists.org
Is Boiling Water A Chemical Or Physical Change What Is Water S Highest Boiling Point The boiling point of a liquid varies according to the applied pressure; However, in this section we learn that the boiling point of a liquid depends on pressure. Impurities in water, like salt,. The high boiling point of water, which is 100°c (212°f) at standard atmospheric pressure, is a result of several factors, including the. It is well known that. What Is Water S Highest Boiling Point.
From sciencenotes.org
What Are the Bubbles in Boiling Water? What Is Water S Highest Boiling Point The boiling point of a liquid varies according to the applied pressure; However, in this section we learn that the boiling point of a liquid depends on pressure. It is well known that boiling point depends on temperature. The normal boiling point is the temperature at which the vapour pressure is equal to. The high boiling point of water, which. What Is Water S Highest Boiling Point.
From exyigmeig.blob.core.windows.net
What Is Water's Normal Boiling Point at Lola Drummer blog What Is Water S Highest Boiling Point The high boiling point of water, which is 100°c (212°f) at standard atmospheric pressure, is a result of several factors, including the. However, in this section we learn that the boiling point of a liquid depends on pressure. The normal boiling point is the temperature at which the vapour pressure is equal to. Most know that water boils at 100. What Is Water S Highest Boiling Point.
From www.thespruceeats.com
The Boiling Point of Water at Various Altitudes What Is Water S Highest Boiling Point Most know that water boils at 100 degrees celsius. However, in this section we learn that the boiling point of a liquid depends on pressure. The boiling point for water is 212 ºf or 100 ºc, whereas the boiling point of salt water is about 102 ºc. Impurities in water, like salt,. The boiling point of a liquid varies according. What Is Water S Highest Boiling Point.
From www.dreamstime.com
Boiling and Evaporation, Freezing and Melting Points of Water Stock What Is Water S Highest Boiling Point The boiling point of a liquid varies according to the applied pressure; The normal boiling point is the temperature at which the vapour pressure is equal to. Impurities in water, like salt,. It is well known that boiling point depends on temperature. Most know that water boils at 100 degrees celsius. However, in this section we learn that the boiling. What Is Water S Highest Boiling Point.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? What Is Water S Highest Boiling Point The boiling point of a liquid varies according to the applied pressure; It is well known that boiling point depends on temperature. Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. The normal boiling point is the temperature at which the vapour pressure is. What Is Water S Highest Boiling Point.
From aweseas.blogspot.com
Boiling Point Of Water At Sea Level In Kelvin What Is Water S Highest Boiling Point However, in this section we learn that the boiling point of a liquid depends on pressure. The boiling point of a liquid varies according to the applied pressure; Most know that water boils at 100 degrees celsius. The high boiling point of water, which is 100°c (212°f) at standard atmospheric pressure, is a result of several factors, including the. The. What Is Water S Highest Boiling Point.
From ceghryvh.blob.core.windows.net
What Is Boiling Point Sea Level at Edwin Acosta blog What Is Water S Highest Boiling Point Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. The normal boiling point is the temperature at which the vapour pressure is equal to. Impurities in water, like salt,. The boiling point of a liquid varies according to the applied pressure; Most know that. What Is Water S Highest Boiling Point.
From www.animalia-life.club
Boiling Point Of Water Examples What Is Water S Highest Boiling Point The high boiling point of water, which is 100°c (212°f) at standard atmospheric pressure, is a result of several factors, including the. Impurities in water, like salt,. The boiling point for water is 212 ºf or 100 ºc, whereas the boiling point of salt water is about 102 ºc. The boiling point of a liquid varies according to the applied. What Is Water S Highest Boiling Point.
From exyqxtcdu.blob.core.windows.net
What Is The Boiling Point Constant Of Water at Beatrice Hughes blog What Is Water S Highest Boiling Point Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. It is well known that boiling point depends on temperature. The high boiling point of water, which is 100°c (212°f) at standard atmospheric pressure, is a result of several factors, including the. The boiling point. What Is Water S Highest Boiling Point.
From exyigmeig.blob.core.windows.net
What Is Water's Normal Boiling Point at Lola Drummer blog What Is Water S Highest Boiling Point Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. The normal boiling point is the temperature at which the vapour pressure is equal to. However, in this section we learn that the boiling point of a liquid depends on pressure. The high boiling point. What Is Water S Highest Boiling Point.
From www.animalia-life.club
Boiling Point Of Water For Kids What Is Water S Highest Boiling Point However, in this section we learn that the boiling point of a liquid depends on pressure. Impurities in water, like salt,. The high boiling point of water, which is 100°c (212°f) at standard atmospheric pressure, is a result of several factors, including the. The normal boiling point is the temperature at which the vapour pressure is equal to. The boiling. What Is Water S Highest Boiling Point.
From engineeringstuff.co.in
What is Boiling point Engineeringstuff What Is Water S Highest Boiling Point Impurities in water, like salt,. Most know that water boils at 100 degrees celsius. The normal boiling point is the temperature at which the vapour pressure is equal to. Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. However, in this section we learn. What Is Water S Highest Boiling Point.
From www.slideserve.com
PPT Water as a Polar Molecule PowerPoint Presentation, free download What Is Water S Highest Boiling Point The normal boiling point is the temperature at which the vapour pressure is equal to. Most know that water boils at 100 degrees celsius. It is well known that boiling point depends on temperature. The boiling point for water is 212 ºf or 100 ºc, whereas the boiling point of salt water is about 102 ºc. However, in this section. What Is Water S Highest Boiling Point.
From gamesmartz.com
Boiling Point Definition & Image GameSmartz What Is Water S Highest Boiling Point It is well known that boiling point depends on temperature. However, in this section we learn that the boiling point of a liquid depends on pressure. The normal boiling point is the temperature at which the vapour pressure is equal to. The boiling point of a liquid varies according to the applied pressure; Most know that water boils at 100. What Is Water S Highest Boiling Point.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples What Is Water S Highest Boiling Point The high boiling point of water, which is 100°c (212°f) at standard atmospheric pressure, is a result of several factors, including the. The boiling point for water is 212 ºf or 100 ºc, whereas the boiling point of salt water is about 102 ºc. Most know that water boils at 100 degrees celsius. However, in this section we learn that. What Is Water S Highest Boiling Point.