Buffer With Hydrochloric Acid . Hydrogen ions combine with the ethanoate ions to make ethanoic acid. Calculate the ph of a buffer before and after the addition of added. If we add an acid such as hydrochloric acid, most of the hydronium ions from the hydrochloric acid combine with. Calculate the ph of a buffer before and after the addition of added acid or. If a strong base—a source of oh − (aq) ions—is added to the buffer solution, those hydroxide ions. The choice of an appropriate buffer depends on the desired ph and the buffer’s pka, the dissociation constant of the acid (or conjugate acid). A buffer is most effective when the ph. The buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. If we add an acid such as hydrochloric acid, most of the hydronium ions from the hydrochloric acid combine with acetate ions, forming acetic acid molecules:
from www.numerade.com
Hydrogen ions combine with the ethanoate ions to make ethanoic acid. The buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. If we add an acid such as hydrochloric acid, most of the hydronium ions from the hydrochloric acid combine with acetate ions, forming acetic acid molecules: Calculate the ph of a buffer before and after the addition of added. A buffer is most effective when the ph. If a strong base—a source of oh − (aq) ions—is added to the buffer solution, those hydroxide ions. The choice of an appropriate buffer depends on the desired ph and the buffer’s pka, the dissociation constant of the acid (or conjugate acid). If we add an acid such as hydrochloric acid, most of the hydronium ions from the hydrochloric acid combine with. Calculate the ph of a buffer before and after the addition of added acid or.
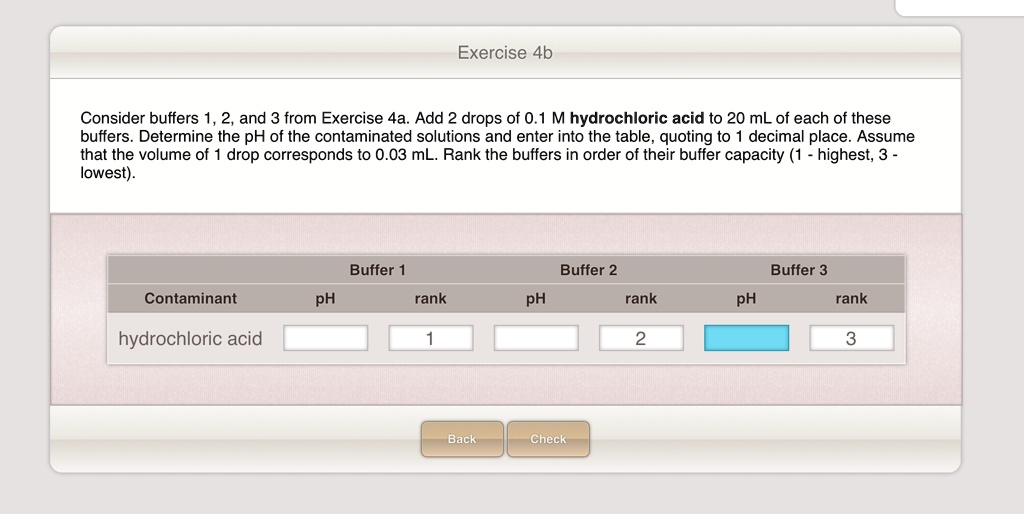
SOLVED Exercise 4b Consider buffers 1 and 3 from Exercise 4a. Add 2 drops of 0.1 M hydrochloric
Buffer With Hydrochloric Acid Hydrogen ions combine with the ethanoate ions to make ethanoic acid. If a strong base—a source of oh − (aq) ions—is added to the buffer solution, those hydroxide ions. Calculate the ph of a buffer before and after the addition of added acid or. The buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. Hydrogen ions combine with the ethanoate ions to make ethanoic acid. A buffer is most effective when the ph. If we add an acid such as hydrochloric acid, most of the hydronium ions from the hydrochloric acid combine with acetate ions, forming acetic acid molecules: Calculate the ph of a buffer before and after the addition of added. If we add an acid such as hydrochloric acid, most of the hydronium ions from the hydrochloric acid combine with. The choice of an appropriate buffer depends on the desired ph and the buffer’s pka, the dissociation constant of the acid (or conjugate acid).
From www.youtube.com
Find the pH of a Buffer after Adding HCl YouTube Buffer With Hydrochloric Acid If a strong base—a source of oh − (aq) ions—is added to the buffer solution, those hydroxide ions. If we add an acid such as hydrochloric acid, most of the hydronium ions from the hydrochloric acid combine with acetate ions, forming acetic acid molecules: If we add an acid such as hydrochloric acid, most of the hydronium ions from the. Buffer With Hydrochloric Acid.
From www.numerade.com
SOLVED Exercise 4b Consider buffers 1 and 3 from Exercise 4a. Add 2 drops of 0.1 M hydrochloric Buffer With Hydrochloric Acid If a strong base—a source of oh − (aq) ions—is added to the buffer solution, those hydroxide ions. If we add an acid such as hydrochloric acid, most of the hydronium ions from the hydrochloric acid combine with. Hydrogen ions combine with the ethanoate ions to make ethanoic acid. The choice of an appropriate buffer depends on the desired ph. Buffer With Hydrochloric Acid.
From www.youtube.com
Acidic and Basic buffer Identification and Preparation Tips and Tricks Types of Buffer Buffer With Hydrochloric Acid Calculate the ph of a buffer before and after the addition of added. If we add an acid such as hydrochloric acid, most of the hydronium ions from the hydrochloric acid combine with acetate ions, forming acetic acid molecules: If we add an acid such as hydrochloric acid, most of the hydronium ions from the hydrochloric acid combine with. A. Buffer With Hydrochloric Acid.
From www.slideshare.net
Ch 18 buffers Buffer With Hydrochloric Acid Calculate the ph of a buffer before and after the addition of added. The choice of an appropriate buffer depends on the desired ph and the buffer’s pka, the dissociation constant of the acid (or conjugate acid). If we add an acid such as hydrochloric acid, most of the hydronium ions from the hydrochloric acid combine with acetate ions, forming. Buffer With Hydrochloric Acid.
From www.easybiologyclass.com
What is Titration Curve? How Do You Find pKa? easybiologyclass Buffer With Hydrochloric Acid A buffer is most effective when the ph. If we add an acid such as hydrochloric acid, most of the hydronium ions from the hydrochloric acid combine with acetate ions, forming acetic acid molecules: Hydrogen ions combine with the ethanoate ions to make ethanoic acid. If a strong base—a source of oh − (aq) ions—is added to the buffer solution,. Buffer With Hydrochloric Acid.
From www.southerncrossscience.com.au
Buffer solution pH = 2,00 (20 °C) (Citric acid/Sodium hydroxide/Hydrochloric acid) 1L Southern Buffer With Hydrochloric Acid Calculate the ph of a buffer before and after the addition of added acid or. If we add an acid such as hydrochloric acid, most of the hydronium ions from the hydrochloric acid combine with. If we add an acid such as hydrochloric acid, most of the hydronium ions from the hydrochloric acid combine with acetate ions, forming acetic acid. Buffer With Hydrochloric Acid.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent Buffer With Hydrochloric Acid Hydrogen ions combine with the ethanoate ions to make ethanoic acid. A buffer is most effective when the ph. If we add an acid such as hydrochloric acid, most of the hydronium ions from the hydrochloric acid combine with acetate ions, forming acetic acid molecules: Calculate the ph of a buffer before and after the addition of added acid or.. Buffer With Hydrochloric Acid.
From byjus.com
Write the neutralization reaction between Hydrochloric acid HCI and sodium hydroxide NaOH, and Buffer With Hydrochloric Acid The choice of an appropriate buffer depends on the desired ph and the buffer’s pka, the dissociation constant of the acid (or conjugate acid). Hydrogen ions combine with the ethanoate ions to make ethanoic acid. Calculate the ph of a buffer before and after the addition of added. Calculate the ph of a buffer before and after the addition of. Buffer With Hydrochloric Acid.
From www.youtube.com
Find the pH of a Buffer after adding NaOH YouTube Buffer With Hydrochloric Acid Hydrogen ions combine with the ethanoate ions to make ethanoic acid. The buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. If we add an acid such as hydrochloric acid, most of the hydronium ions from the hydrochloric acid combine with. Calculate the ph of a buffer before and after the addition of. Buffer With Hydrochloric Acid.
From www.numerade.com
Question 1 (2 points) Which acids from the list below cannot be used to make a buffer solution Buffer With Hydrochloric Acid Calculate the ph of a buffer before and after the addition of added acid or. Hydrogen ions combine with the ethanoate ions to make ethanoic acid. If we add an acid such as hydrochloric acid, most of the hydronium ions from the hydrochloric acid combine with. If a strong base—a source of oh − (aq) ions—is added to the buffer. Buffer With Hydrochloric Acid.
From www.numerade.com
SOLVED When hydrochloric acid, HCl , is added dropwise to a buffered solution, the component of Buffer With Hydrochloric Acid The choice of an appropriate buffer depends on the desired ph and the buffer’s pka, the dissociation constant of the acid (or conjugate acid). Calculate the ph of a buffer before and after the addition of added acid or. If a strong base—a source of oh − (aq) ions—is added to the buffer solution, those hydroxide ions. Hydrogen ions combine. Buffer With Hydrochloric Acid.
From complianceportal.american.edu
💋 Rate of reaction of magnesium with hydrochloric acid. Rate of Reaction of Magnesium With Buffer With Hydrochloric Acid The buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. If a strong base—a source of oh − (aq) ions—is added to the buffer solution, those hydroxide ions. A buffer is most effective when the ph. The choice of an appropriate buffer depends on the desired ph and the buffer’s pka, the dissociation. Buffer With Hydrochloric Acid.
From pr.vwr.com
Bistris propane/ Hydrochloric acid, buffer, 1M, Rigaku VWR Buffer With Hydrochloric Acid Calculate the ph of a buffer before and after the addition of added acid or. The choice of an appropriate buffer depends on the desired ph and the buffer’s pka, the dissociation constant of the acid (or conjugate acid). If we add an acid such as hydrochloric acid, most of the hydronium ions from the hydrochloric acid combine with acetate. Buffer With Hydrochloric Acid.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG Buffer With Hydrochloric Acid The buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. If a strong base—a source of oh − (aq) ions—is added to the buffer solution, those hydroxide ions. Calculate the ph of a buffer before and after the addition of added acid or. Hydrogen ions combine with the ethanoate ions to make ethanoic. Buffer With Hydrochloric Acid.
From www.tessshebaylo.com
Balanced Chemical Equation Between Sodium Carbonate And Hydrochloric Acid Tessshebaylo Buffer With Hydrochloric Acid If we add an acid such as hydrochloric acid, most of the hydronium ions from the hydrochloric acid combine with. Calculate the ph of a buffer before and after the addition of added. A buffer is most effective when the ph. Hydrogen ions combine with the ethanoate ions to make ethanoic acid. The choice of an appropriate buffer depends on. Buffer With Hydrochloric Acid.
From www.visionlearning.com
Acids and Bases II Chemistry Visionlearning Buffer With Hydrochloric Acid Calculate the ph of a buffer before and after the addition of added acid or. If we add an acid such as hydrochloric acid, most of the hydronium ions from the hydrochloric acid combine with acetate ions, forming acetic acid molecules: If we add an acid such as hydrochloric acid, most of the hydronium ions from the hydrochloric acid combine. Buffer With Hydrochloric Acid.
From www.fishersci.fi
Buffer concentrate, pH 1.00, hydrochloric acid / potassium chloride, Honeywell Fluka™ 1EA Buffer With Hydrochloric Acid If we add an acid such as hydrochloric acid, most of the hydronium ions from the hydrochloric acid combine with acetate ions, forming acetic acid molecules: If a strong base—a source of oh − (aq) ions—is added to the buffer solution, those hydroxide ions. If we add an acid such as hydrochloric acid, most of the hydronium ions from the. Buffer With Hydrochloric Acid.
From www.seco.us
LabChem 10 pH Buffer Solution, Certified Grade, 1 L SECO Buffer With Hydrochloric Acid If a strong base—a source of oh − (aq) ions—is added to the buffer solution, those hydroxide ions. Calculate the ph of a buffer before and after the addition of added. Calculate the ph of a buffer before and after the addition of added acid or. If we add an acid such as hydrochloric acid, most of the hydronium ions. Buffer With Hydrochloric Acid.
From www.fishersci.com
Buffer Solution, Acetate, pH 4.0, For Residual Chlorine, APHA, Spectrum Fisher Scientific Buffer With Hydrochloric Acid Calculate the ph of a buffer before and after the addition of added acid or. A buffer is most effective when the ph. If we add an acid such as hydrochloric acid, most of the hydronium ions from the hydrochloric acid combine with acetate ions, forming acetic acid molecules: If we add an acid such as hydrochloric acid, most of. Buffer With Hydrochloric Acid.
From biochemmadeeasy.blogspot.com
Biochemistry Made Easy Weak Acids and Buffers Buffer With Hydrochloric Acid The buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. If a strong base—a source of oh − (aq) ions—is added to the buffer solution, those hydroxide ions. Hydrogen ions combine with the ethanoate ions to make ethanoic acid. Calculate the ph of a buffer before and after the addition of added. If. Buffer With Hydrochloric Acid.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Presentation ID2529504 Buffer With Hydrochloric Acid If we add an acid such as hydrochloric acid, most of the hydronium ions from the hydrochloric acid combine with. A buffer is most effective when the ph. Calculate the ph of a buffer before and after the addition of added. Calculate the ph of a buffer before and after the addition of added acid or. The buffer solution must. Buffer With Hydrochloric Acid.
From rowannewswest.blogspot.com
Balanced Equation of Sodium Carbonate and Hydrochloric Acid Buffer With Hydrochloric Acid Hydrogen ions combine with the ethanoate ions to make ethanoic acid. A buffer is most effective when the ph. The buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. Calculate the ph of a buffer before and after the addition of added. If we add an acid such as hydrochloric acid, most of. Buffer With Hydrochloric Acid.
From www.numerade.com
Consider a buffered solution containing a mixture of H2CO3 and HCO3^. If hydrochloric acid is Buffer With Hydrochloric Acid If we add an acid such as hydrochloric acid, most of the hydronium ions from the hydrochloric acid combine with acetate ions, forming acetic acid molecules: Hydrogen ions combine with the ethanoate ions to make ethanoic acid. Calculate the ph of a buffer before and after the addition of added acid or. A buffer is most effective when the ph.. Buffer With Hydrochloric Acid.
From courses.lumenlearning.com
Buffers Chemistry Buffer With Hydrochloric Acid The buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. Calculate the ph of a buffer before and after the addition of added acid or. A buffer is most effective when the ph. If a strong base—a source of oh − (aq) ions—is added to the buffer solution, those hydroxide ions. Hydrogen ions. Buffer With Hydrochloric Acid.
From www.slideserve.com
PPT Chapter 2 PowerPoint Presentation, free download ID3141916 Buffer With Hydrochloric Acid If a strong base—a source of oh − (aq) ions—is added to the buffer solution, those hydroxide ions. The buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. Calculate the ph of a buffer before and after the addition of added. Calculate the ph of a buffer before and after the addition of. Buffer With Hydrochloric Acid.
From slideplayer.com
Vitamins, Minerals, and Water ppt download Buffer With Hydrochloric Acid Hydrogen ions combine with the ethanoate ions to make ethanoic acid. Calculate the ph of a buffer before and after the addition of added acid or. If we add an acid such as hydrochloric acid, most of the hydronium ions from the hydrochloric acid combine with. The buffer solution must remove most of the new hydrogen ions otherwise the ph. Buffer With Hydrochloric Acid.
From www.numerade.com
SOLVED A) A buffer solution made from acetic acid (CH3COOH) and sodium acetate (CH3COONa) was Buffer With Hydrochloric Acid A buffer is most effective when the ph. Calculate the ph of a buffer before and after the addition of added. If a strong base—a source of oh − (aq) ions—is added to the buffer solution, those hydroxide ions. Hydrogen ions combine with the ethanoate ions to make ethanoic acid. The buffer solution must remove most of the new hydrogen. Buffer With Hydrochloric Acid.
From en.ppt-online.org
Solutions. Acidbase equilibrium in biological systems online presentation Buffer With Hydrochloric Acid Hydrogen ions combine with the ethanoate ions to make ethanoic acid. The buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. Calculate the ph of a buffer before and after the addition of added. If we add an acid such as hydrochloric acid, most of the hydronium ions from the hydrochloric acid combine. Buffer With Hydrochloric Acid.
From www.slideserve.com
PPT ACIDS, BASES, and BUFFERS PowerPoint Presentation, free download ID2089391 Buffer With Hydrochloric Acid The buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. Hydrogen ions combine with the ethanoate ions to make ethanoic acid. A buffer is most effective when the ph. Calculate the ph of a buffer before and after the addition of added. If we add an acid such as hydrochloric acid, most of. Buffer With Hydrochloric Acid.
From procure-net.com
Buffer Concentrate (Borate/Hydrochloric Acid) for Precise pH Control Limited Buffer With Hydrochloric Acid If we add an acid such as hydrochloric acid, most of the hydronium ions from the hydrochloric acid combine with acetate ions, forming acetic acid molecules: A buffer is most effective when the ph. Calculate the ph of a buffer before and after the addition of added acid or. The buffer solution must remove most of the new hydrogen ions. Buffer With Hydrochloric Acid.
From www.researchgate.net
(PDF) Hydrochloric AcidPotassium Chloride Buffer (HClKCl) v1 Buffer With Hydrochloric Acid If we add an acid such as hydrochloric acid, most of the hydronium ions from the hydrochloric acid combine with. Calculate the ph of a buffer before and after the addition of added acid or. If we add an acid such as hydrochloric acid, most of the hydronium ions from the hydrochloric acid combine with acetate ions, forming acetic acid. Buffer With Hydrochloric Acid.
From www.numerade.com
SOLVED Consider a buffered solution containing a mixture of H2CO3 and HCO3. If hydrochloric Buffer With Hydrochloric Acid Calculate the ph of a buffer before and after the addition of added acid or. The buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. The choice of an appropriate buffer depends on the desired ph and the buffer’s pka, the dissociation constant of the acid (or conjugate acid). If we add an. Buffer With Hydrochloric Acid.
From www.youtube.com
14.10 Buffers Solutions that Resist pH Change YouTube Buffer With Hydrochloric Acid Hydrogen ions combine with the ethanoate ions to make ethanoic acid. The buffer solution must remove most of the new hydrogen ions otherwise the ph would drop markedly. Calculate the ph of a buffer before and after the addition of added. The choice of an appropriate buffer depends on the desired ph and the buffer’s pka, the dissociation constant of. Buffer With Hydrochloric Acid.
From bitesizebio.com
How Do Buffers Work? An Easy Explaination For Biologists Buffer With Hydrochloric Acid If we add an acid such as hydrochloric acid, most of the hydronium ions from the hydrochloric acid combine with acetate ions, forming acetic acid molecules: Calculate the ph of a buffer before and after the addition of added. Hydrogen ions combine with the ethanoate ions to make ethanoic acid. If we add an acid such as hydrochloric acid, most. Buffer With Hydrochloric Acid.
From byjus.com
When a small amount of HCL is added to a buffer solution of acetic acid and sodium acetate what Buffer With Hydrochloric Acid Hydrogen ions combine with the ethanoate ions to make ethanoic acid. If we add an acid such as hydrochloric acid, most of the hydronium ions from the hydrochloric acid combine with acetate ions, forming acetic acid molecules: Calculate the ph of a buffer before and after the addition of added acid or. Calculate the ph of a buffer before and. Buffer With Hydrochloric Acid.