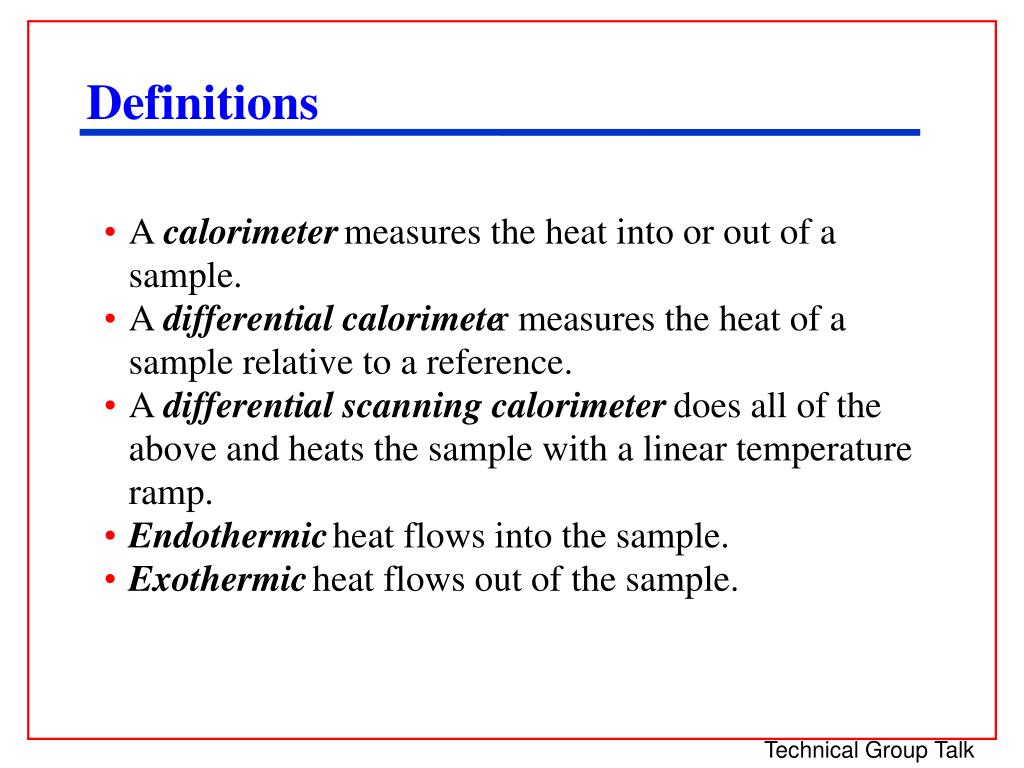
Difference Between Calorimetry And Enthalpy . At constant v, pδv term was zero, giving. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Calorimetry is used to measure amounts of heat transferred to or from a substance. In both cases you should be able to. Enthalpy changes of reactions in solution. Q + w = q. There are two types of calorimetry experiments for you to know: Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. In particular, if gases are involved, pv = nrt. If p is constant and δt ≠ 0, then δv ≠ 0. How does this relate to ∆u, etc.? To do so, the heat is exchanged with a calibrated object. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is.
from www.slideserve.com
Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount. Q + w = q. In both cases you should be able to. Enthalpy changes of reactions in solution. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. If p is constant and δt ≠ 0, then δv ≠ 0. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. There are two types of calorimetry experiments for you to know: A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends.
PPT Differential Scanning Calorimetry PowerPoint Presentation, free download ID4258715
Difference Between Calorimetry And Enthalpy There are two types of calorimetry experiments for you to know: In both cases you should be able to. Enthalpy changes of reactions in solution. In particular, if gases are involved, pv = nrt. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. How does this relate to ∆u, etc.? Calorimetry is used to measure amounts of heat transferred to or from a substance. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. There are two types of calorimetry experiments for you to know: Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount. To do so, the heat is exchanged with a calibrated object. If p is constant and δt ≠ 0, then δv ≠ 0. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. At constant v, pδv term was zero, giving. Q + w = q.
From pediaa.com
What is the Difference Between Enthalpy of Formation and Enthalpy of Reaction Difference Between Calorimetry And Enthalpy In particular, if gases are involved, pv = nrt. If p is constant and δt ≠ 0, then δv ≠ 0. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. How does this relate to ∆u, etc.? Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature. Difference Between Calorimetry And Enthalpy.
From studylib.net
Calorimetry & Enthalpy Changes Difference Between Calorimetry And Enthalpy Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. To do so, the heat is exchanged with a calibrated object. Enthalpy changes of reactions in solution. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Calorimetry measures enthalpy changes during chemical processes, where the. Difference Between Calorimetry And Enthalpy.
From wisc.pb.unizin.org
Calorimetry continued Types of Calorimeters and Analyzing Heat Flow (M6Q5) UWMadison Difference Between Calorimetry And Enthalpy How does this relate to ∆u, etc.? To do so, the heat is exchanged with a calibrated object. If p is constant and δt ≠ 0, then δv ≠ 0. Q + w = q. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. In particular, if gases are involved, pv = nrt. A. Difference Between Calorimetry And Enthalpy.
From slidetodoc.com
CALORIMETRY ENTHALPY CHANGES be able to define enthalpy Difference Between Calorimetry And Enthalpy Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Calorimetry is used to measure amounts of heat transferred to or from a substance. If p is constant and δt ≠ 0, then δv ≠ 0. In both cases you should be able to. Enthalpy changes of reactions in solution. At constant v, pδv term. Difference Between Calorimetry And Enthalpy.
From dokumen.tips
(PPT) Enthalpy changes and calorimetry Enthalpy changes in reactions Calorimetry and heat Difference Between Calorimetry And Enthalpy Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. In particular, if gases are involved, pv = nrt. Q + w = q. In both cases you should be able to. If p is constant and δt ≠ 0, then δv ≠ 0. There are two types of calorimetry experiments for you to know:. Difference Between Calorimetry And Enthalpy.
From www.studocu.com
Enthalpy and Calorimetry lesson 4 Enthalpies of Reaction & Calorimetry calorimetry Difference Between Calorimetry And Enthalpy A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. There are two types of calorimetry experiments for you to know: Q + w = q. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. Difference Between Calorimetry And Enthalpy.
From www.slideserve.com
PPT AS MODULE CHEM2 ENERGETICS PowerPoint Presentation, free download ID4388846 Difference Between Calorimetry And Enthalpy Calorimetry is used to measure amounts of heat transferred to or from a substance. If p is constant and δt ≠ 0, then δv ≠ 0. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. At constant v, pδv term was zero, giving. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of. Difference Between Calorimetry And Enthalpy.
From www.differencebetween.com
Difference Between Enthalpy and Heat Compare the Difference Between Similar Terms Difference Between Calorimetry And Enthalpy At constant v, pδv term was zero, giving. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount. If p is constant and δt ≠ 0, then δv ≠ 0. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. To do so, the heat is exchanged. Difference Between Calorimetry And Enthalpy.
From www.slideshare.net
Lesson Enthalpy and Calorimetry Difference Between Calorimetry And Enthalpy Enthalpy changes of reactions in solution. At constant v, pδv term was zero, giving. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the. Difference Between Calorimetry And Enthalpy.
From www.slideshare.net
Lesson Enthalpy and Calorimetry Difference Between Calorimetry And Enthalpy There are two types of calorimetry experiments for you to know: Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Enthalpy changes of reactions in solution. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. To do so, the heat is exchanged with a calibrated object. Calorimetry measures. Difference Between Calorimetry And Enthalpy.
From www.slideshare.net
Lesson Enthalpy and Calorimetry Difference Between Calorimetry And Enthalpy How does this relate to ∆u, etc.? In particular, if gases are involved, pv = nrt. To do so, the heat is exchanged with a calibrated object. Enthalpy changes of reactions in solution. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific. Difference Between Calorimetry And Enthalpy.
From studylib.net
PPT Calorimetry Difference Between Calorimetry And Enthalpy How does this relate to ∆u, etc.? Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. There are two types of calorimetry experiments for you to know: Enthalpy changes of reactions in solution. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry measures enthalpy changes during chemical processes,. Difference Between Calorimetry And Enthalpy.
From www.youtube.com
Using Calorimetry to Calculate Enthalpies of Reaction Chemistry Tutorial YouTube Difference Between Calorimetry And Enthalpy Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. In both cases you should be able to. Q + w = q. At constant v, pδv term was zero, giving. How does this relate to ∆u, etc.? There are two types of calorimetry experiments for you to know: Enthalpy changes of reactions in solution.. Difference Between Calorimetry And Enthalpy.
From www.slideserve.com
PPT AS MODULE CHEM2 ENERGETICS PowerPoint Presentation, free download ID4388846 Difference Between Calorimetry And Enthalpy There are two types of calorimetry experiments for you to know: Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. In particular, if gases are involved, pv = nrt. Q + w = q. Calorimetry. Difference Between Calorimetry And Enthalpy.
From www.youtube.com
Difference between Enthalpy and Energy, bomb calorimeter and coffee cup calorimeter, Thermo Difference Between Calorimetry And Enthalpy Enthalpy changes of reactions in solution. In both cases you should be able to. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. There are two types of calorimetry experiments for you to know: Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on. Difference Between Calorimetry And Enthalpy.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID2854293 Difference Between Calorimetry And Enthalpy Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. At constant v, pδv term was zero, giving. If p is constant and δt ≠ 0, then δv ≠ 0. Q + w = q. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to. Difference Between Calorimetry And Enthalpy.
From www.slideshare.net
Lesson Enthalpy and Calorimetry Difference Between Calorimetry And Enthalpy At constant v, pδv term was zero, giving. If p is constant and δt ≠ 0, then δv ≠ 0. In particular, if gases are involved, pv = nrt. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. In. Difference Between Calorimetry And Enthalpy.
From www.slideshare.net
Lesson Enthalpy and Calorimetry Difference Between Calorimetry And Enthalpy Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. At constant v, pδv term was zero, giving. There are two types of calorimetry experiments for you to know: Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount. If p is constant and δt ≠ 0,. Difference Between Calorimetry And Enthalpy.
From www.slideshare.net
Lesson Enthalpy and Calorimetry Difference Between Calorimetry And Enthalpy Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. There are two types of calorimetry experiments for you to know: Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Enthalpy changes of reactions in solution. How does this relate to ∆u, etc.? At constant v, pδv term was. Difference Between Calorimetry And Enthalpy.
From pathtowarren.com
What is the difference between entropy and enthalpy? Path To Warren Difference Between Calorimetry And Enthalpy Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Enthalpy changes of reactions in solution. In both cases you should be able to. To do so, the heat is exchanged with a calibrated object. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Q + w = q.. Difference Between Calorimetry And Enthalpy.
From slideplayer.com
Calorimetry CP Unit 9 Chapter ppt download Difference Between Calorimetry And Enthalpy How does this relate to ∆u, etc.? Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount. To do so, the heat is exchanged with a calibrated object. At constant v, pδv term was zero, giving. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. A. Difference Between Calorimetry And Enthalpy.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle and Structure of Difference Between Calorimetry And Enthalpy Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends.. Difference Between Calorimetry And Enthalpy.
From www.scribd.com
Possible Origin of Differences Between Vant Hoff and Calorimetric Enthalpy Estimates PDF Difference Between Calorimetry And Enthalpy A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. How does this relate to ∆u, etc.? Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Q + w = q. Calorimetry is used. Difference Between Calorimetry And Enthalpy.
From studylib.net
calorimetryandenthalpyofreaction Difference Between Calorimetry And Enthalpy In particular, if gases are involved, pv = nrt. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. Enthalpy changes of reactions in solution. Q + w = q. At constant v, pδv term was zero, giving. Calorimetry is used to measure amounts of heat transferred to or from a. Difference Between Calorimetry And Enthalpy.
From www.slideshare.net
Lesson Enthalpy and Calorimetry Difference Between Calorimetry And Enthalpy A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. There are two types of calorimetry experiments for you to know: Enthalpy changes of reactions in solution. In both cases you should be able to. Q + w = q.. Difference Between Calorimetry And Enthalpy.
From slidetodoc.com
CALORIMETRY ENTHALPY CHANGES be able to define enthalpy Difference Between Calorimetry And Enthalpy In particular, if gases are involved, pv = nrt. In both cases you should be able to. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount. If p is constant and δt ≠ 0, then δv ≠ 0. How does this relate to ∆u, etc.? Q + w = q. At. Difference Between Calorimetry And Enthalpy.
From www.pw.live
Difference Between Enthalpy And Entropy, Definition, Applications Difference Between Calorimetry And Enthalpy How does this relate to ∆u, etc.? Enthalpy changes of reactions in solution. At constant v, pδv term was zero, giving. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. Calorimetry measures enthalpy changes during chemical processes, where the. Difference Between Calorimetry And Enthalpy.
From pediaa.com
Difference Between Enthalpy and Internal Energy Definition, Units, Formula for Calculation Difference Between Calorimetry And Enthalpy Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. How does this relate to ∆u, etc.? In particular, if gases are involved, pv = nrt. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount. Q + w = q. At constant v, pδv term was. Difference Between Calorimetry And Enthalpy.
From slidetodoc.com
CALORIMETRY ENTHALPY CHANGES be able to define enthalpy Difference Between Calorimetry And Enthalpy There are two types of calorimetry experiments for you to know: If p is constant and δt ≠ 0, then δv ≠ 0. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry measures enthalpy changes. Difference Between Calorimetry And Enthalpy.
From www.slideshare.net
Lesson Enthalpy and Calorimetry Difference Between Calorimetry And Enthalpy Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. At constant v, pδv term was zero, giving. If p is constant and δt ≠. Difference Between Calorimetry And Enthalpy.
From www.slideshare.net
Lesson Enthalpy and Calorimetry Difference Between Calorimetry And Enthalpy How does this relate to ∆u, etc.? Enthalpy changes of reactions in solution. In both cases you should be able to. To do so, the heat is exchanged with a calibrated object. Q + w = q. If p is constant and δt ≠ 0, then δv ≠ 0. At constant v, pδv term was zero, giving. There are two. Difference Between Calorimetry And Enthalpy.
From www.slideshare.net
Lesson Enthalpy and Calorimetry Difference Between Calorimetry And Enthalpy There are two types of calorimetry experiments for you to know: A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. In particular, if gases are involved, pv = nrt. At constant v, pδv term was zero, giving. In both. Difference Between Calorimetry And Enthalpy.
From www.slideserve.com
PPT Differential Scanning Calorimetry PowerPoint Presentation, free download ID4258715 Difference Between Calorimetry And Enthalpy At constant v, pδv term was zero, giving. To do so, the heat is exchanged with a calibrated object. How does this relate to ∆u, etc.? Calorimetry is used to measure amounts of heat transferred to or from a substance. Q + w = q. A container that prevents heat transfer in or out is called a calorimeter, and the. Difference Between Calorimetry And Enthalpy.
From slidetodoc.com
CALORIMETRY ENTHALPY CHANGES be able to define enthalpy Difference Between Calorimetry And Enthalpy Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. In particular, if gases are involved, pv = nrt. If p is. Difference Between Calorimetry And Enthalpy.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID2854293 Difference Between Calorimetry And Enthalpy Q + w = q. If p is constant and δt ≠ 0, then δv ≠ 0. To do so, the heat is exchanged with a calibrated object. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. Enthalpy changes. Difference Between Calorimetry And Enthalpy.