Hepatitis B Cure News . Gsk plc announced today that the us food and drug administration (fda) has granted fast track designation for bepirovirsen, an investigational antisense oligonucleotide (aso) for the treatment of chronic hepatitis b (chb). The new nih plan defines a hepatitis b “cure” as a sustained loss of a specific protein on the surface of hbv called hepatitis b virus surface antigen — preferably with antibodies against the antigen and undetectable viral dna after completion of a finite course of treatment. The biggest unmet need for hepatitis b patients is a cure, but there are a number of trials looking to achieve functional cure for the disease. Gsk’s bepirovirsen, which is looking for a functional cure, showed very promising results during a phase ii readout in june 2022 and has now initiated a phase iii trial due to end in 2025. Maintaining momentum in developing such strategies will require continued investment. Innovative curative strategies could reshape the treatment of chronic hepatitis b. A functional cure is said to have been achieved if a biomarker called hepatitis b surface antigen (hbsag) is cleared from the blood and remains undetectable after stopping all treatment. Italian researchers have developed a combination of a drug and an antibody that triggers specific immune cells to fight the hepatitis virus, preventing it from damaging.
from www.frontiersin.org
A functional cure is said to have been achieved if a biomarker called hepatitis b surface antigen (hbsag) is cleared from the blood and remains undetectable after stopping all treatment. Italian researchers have developed a combination of a drug and an antibody that triggers specific immune cells to fight the hepatitis virus, preventing it from damaging. Maintaining momentum in developing such strategies will require continued investment. Gsk plc announced today that the us food and drug administration (fda) has granted fast track designation for bepirovirsen, an investigational antisense oligonucleotide (aso) for the treatment of chronic hepatitis b (chb). Gsk’s bepirovirsen, which is looking for a functional cure, showed very promising results during a phase ii readout in june 2022 and has now initiated a phase iii trial due to end in 2025. The new nih plan defines a hepatitis b “cure” as a sustained loss of a specific protein on the surface of hbv called hepatitis b virus surface antigen — preferably with antibodies against the antigen and undetectable viral dna after completion of a finite course of treatment. The biggest unmet need for hepatitis b patients is a cure, but there are a number of trials looking to achieve functional cure for the disease. Innovative curative strategies could reshape the treatment of chronic hepatitis b.
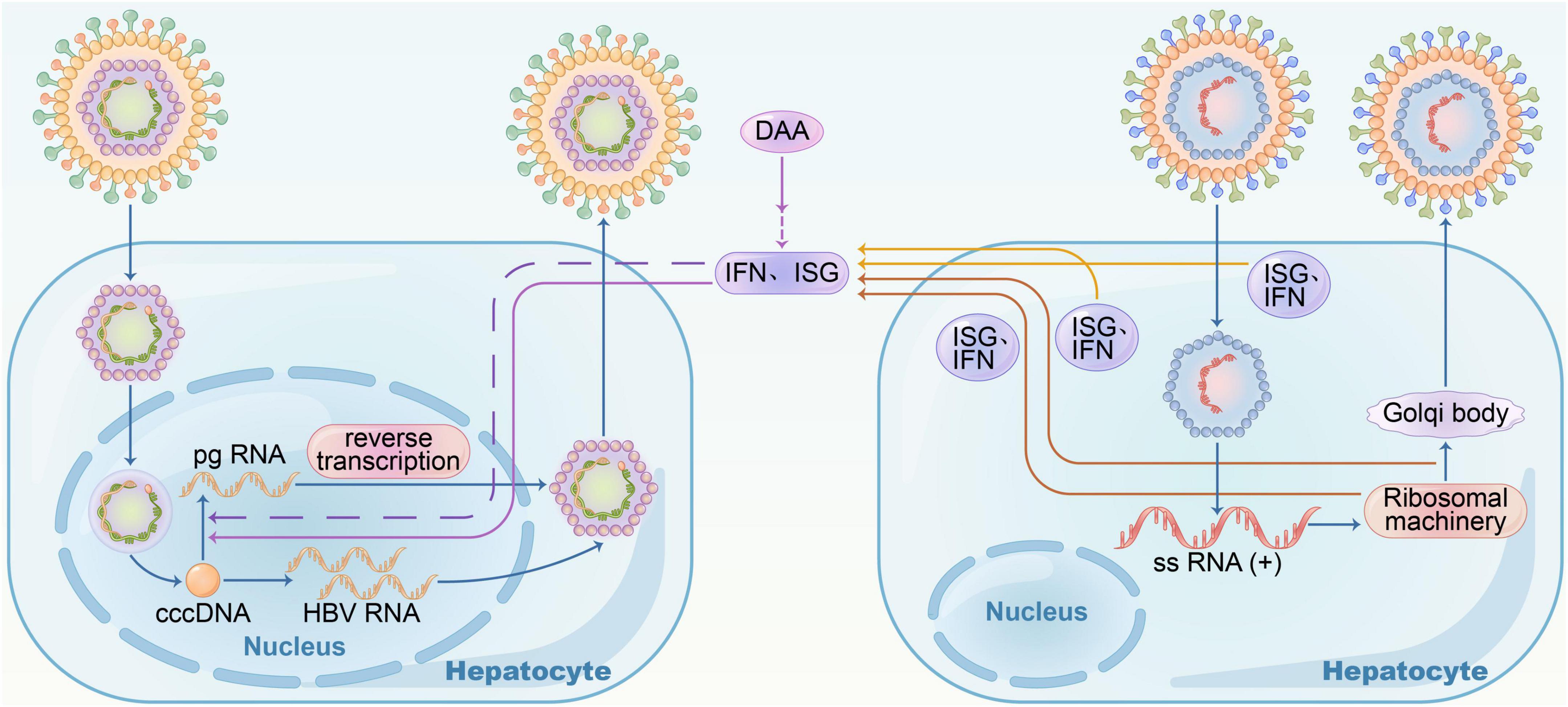
Frontiers Current treatment of chronic hepatitis B Clinical aspects and future directions
Hepatitis B Cure News Gsk’s bepirovirsen, which is looking for a functional cure, showed very promising results during a phase ii readout in june 2022 and has now initiated a phase iii trial due to end in 2025. Maintaining momentum in developing such strategies will require continued investment. The biggest unmet need for hepatitis b patients is a cure, but there are a number of trials looking to achieve functional cure for the disease. Italian researchers have developed a combination of a drug and an antibody that triggers specific immune cells to fight the hepatitis virus, preventing it from damaging. Gsk’s bepirovirsen, which is looking for a functional cure, showed very promising results during a phase ii readout in june 2022 and has now initiated a phase iii trial due to end in 2025. A functional cure is said to have been achieved if a biomarker called hepatitis b surface antigen (hbsag) is cleared from the blood and remains undetectable after stopping all treatment. The new nih plan defines a hepatitis b “cure” as a sustained loss of a specific protein on the surface of hbv called hepatitis b virus surface antigen — preferably with antibodies against the antigen and undetectable viral dna after completion of a finite course of treatment. Gsk plc announced today that the us food and drug administration (fda) has granted fast track designation for bepirovirsen, an investigational antisense oligonucleotide (aso) for the treatment of chronic hepatitis b (chb). Innovative curative strategies could reshape the treatment of chronic hepatitis b.
From www.aidsmap.com
Hepatitis B treatment suboptimal in people with HIV in Africa aidsmap Hepatitis B Cure News Italian researchers have developed a combination of a drug and an antibody that triggers specific immune cells to fight the hepatitis virus, preventing it from damaging. The biggest unmet need for hepatitis b patients is a cure, but there are a number of trials looking to achieve functional cure for the disease. A functional cure is said to have been. Hepatitis B Cure News.
From www.mdpi.com
Biomolecules Free FullText Chronic Hepatitis B Infection New Approaches towards Cure Hepatitis B Cure News Maintaining momentum in developing such strategies will require continued investment. Gsk plc announced today that the us food and drug administration (fda) has granted fast track designation for bepirovirsen, an investigational antisense oligonucleotide (aso) for the treatment of chronic hepatitis b (chb). The new nih plan defines a hepatitis b “cure” as a sustained loss of a specific protein on. Hepatitis B Cure News.
From www.hepb.org
Get Vaccinated for Hepatitis B! Hepatitis B Foundation Hepatitis B Cure News Maintaining momentum in developing such strategies will require continued investment. The new nih plan defines a hepatitis b “cure” as a sustained loss of a specific protein on the surface of hbv called hepatitis b virus surface antigen — preferably with antibodies against the antigen and undetectable viral dna after completion of a finite course of treatment. Italian researchers have. Hepatitis B Cure News.
From hepatitiseducation.med.ubc.ca
Hepatitis Education Canada Programme canadien d’éducation sur l’hépatite Hepatitis B Cure News The new nih plan defines a hepatitis b “cure” as a sustained loss of a specific protein on the surface of hbv called hepatitis b virus surface antigen — preferably with antibodies against the antigen and undetectable viral dna after completion of a finite course of treatment. Innovative curative strategies could reshape the treatment of chronic hepatitis b. Gsk plc. Hepatitis B Cure News.
From www.rockefeller.edu
The Rockefeller University » New tool to study hepatitis B could open the door to a cure Hepatitis B Cure News Italian researchers have developed a combination of a drug and an antibody that triggers specific immune cells to fight the hepatitis virus, preventing it from damaging. The new nih plan defines a hepatitis b “cure” as a sustained loss of a specific protein on the surface of hbv called hepatitis b virus surface antigen — preferably with antibodies against the. Hepatitis B Cure News.
From www.hepb.org
Accessing Hepatitis B Treatment Hepatitis B Foundation Hepatitis B Cure News Italian researchers have developed a combination of a drug and an antibody that triggers specific immune cells to fight the hepatitis virus, preventing it from damaging. Gsk plc announced today that the us food and drug administration (fda) has granted fast track designation for bepirovirsen, an investigational antisense oligonucleotide (aso) for the treatment of chronic hepatitis b (chb). The new. Hepatitis B Cure News.
From www.hepb.org
Accessing Hepatitis B Treatment Hepatitis B Foundation Hepatitis B Cure News A functional cure is said to have been achieved if a biomarker called hepatitis b surface antigen (hbsag) is cleared from the blood and remains undetectable after stopping all treatment. Italian researchers have developed a combination of a drug and an antibody that triggers specific immune cells to fight the hepatitis virus, preventing it from damaging. Maintaining momentum in developing. Hepatitis B Cure News.
From www.hepb.org
Why You Should Get the Hepatitis B Vaccine Hepatitis B Foundation Hepatitis B Cure News The biggest unmet need for hepatitis b patients is a cure, but there are a number of trials looking to achieve functional cure for the disease. Innovative curative strategies could reshape the treatment of chronic hepatitis b. Maintaining momentum in developing such strategies will require continued investment. The new nih plan defines a hepatitis b “cure” as a sustained loss. Hepatitis B Cure News.
From www.aarp.org
Who Really Needs the Hepatitis B Vaccine? Hepatitis B Cure News The new nih plan defines a hepatitis b “cure” as a sustained loss of a specific protein on the surface of hbv called hepatitis b virus surface antigen — preferably with antibodies against the antigen and undetectable viral dna after completion of a finite course of treatment. A functional cure is said to have been achieved if a biomarker called. Hepatitis B Cure News.
From www.aasld.org
Hep, Hep, Hepatitis! AASLD Hepatitis B Cure News The new nih plan defines a hepatitis b “cure” as a sustained loss of a specific protein on the surface of hbv called hepatitis b virus surface antigen — preferably with antibodies against the antigen and undetectable viral dna after completion of a finite course of treatment. Gsk plc announced today that the us food and drug administration (fda) has. Hepatitis B Cure News.
From www.mindray.com
The ABC's of Hepatitis know and fight the viral disease Mindray Hepatitis B Cure News Gsk plc announced today that the us food and drug administration (fda) has granted fast track designation for bepirovirsen, an investigational antisense oligonucleotide (aso) for the treatment of chronic hepatitis b (chb). A functional cure is said to have been achieved if a biomarker called hepatitis b surface antigen (hbsag) is cleared from the blood and remains undetectable after stopping. Hepatitis B Cure News.
From www.mdpi.com
JPM Free FullText Current Therapy of Chronic Viral Hepatitis B, C and D Hepatitis B Cure News Italian researchers have developed a combination of a drug and an antibody that triggers specific immune cells to fight the hepatitis virus, preventing it from damaging. The biggest unmet need for hepatitis b patients is a cure, but there are a number of trials looking to achieve functional cure for the disease. A functional cure is said to have been. Hepatitis B Cure News.
From hepatitisc.net
Hepatitis B Reactivation with Hepatitis C Treatment Hepatitis B Cure News Italian researchers have developed a combination of a drug and an antibody that triggers specific immune cells to fight the hepatitis virus, preventing it from damaging. Gsk plc announced today that the us food and drug administration (fda) has granted fast track designation for bepirovirsen, an investigational antisense oligonucleotide (aso) for the treatment of chronic hepatitis b (chb). The new. Hepatitis B Cure News.
From www.verywellhealth.com
How Do I Get Tested for Hepatitis B/HBV? Hepatitis B Cure News A functional cure is said to have been achieved if a biomarker called hepatitis b surface antigen (hbsag) is cleared from the blood and remains undetectable after stopping all treatment. Maintaining momentum in developing such strategies will require continued investment. The biggest unmet need for hepatitis b patients is a cure, but there are a number of trials looking to. Hepatitis B Cure News.
From www.thelancet.com
Targets and future directacting antiviral approaches to achieve hepatitis B virus cure The Hepatitis B Cure News A functional cure is said to have been achieved if a biomarker called hepatitis b surface antigen (hbsag) is cleared from the blood and remains undetectable after stopping all treatment. The biggest unmet need for hepatitis b patients is a cure, but there are a number of trials looking to achieve functional cure for the disease. Gsk plc announced today. Hepatitis B Cure News.
From www.nytimes.com
Harvoni, a Hepatitis C Drug From Gilead, Wins F.D.A. Approval The New York Times Hepatitis B Cure News Gsk plc announced today that the us food and drug administration (fda) has granted fast track designation for bepirovirsen, an investigational antisense oligonucleotide (aso) for the treatment of chronic hepatitis b (chb). The biggest unmet need for hepatitis b patients is a cure, but there are a number of trials looking to achieve functional cure for the disease. Gsk’s bepirovirsen,. Hepatitis B Cure News.
From www.pathkindlabs.com
Comprehensive Guide to Hepatitis B Screening, Diagnosis & Treatment Hepatitis B Cure News The biggest unmet need for hepatitis b patients is a cure, but there are a number of trials looking to achieve functional cure for the disease. Innovative curative strategies could reshape the treatment of chronic hepatitis b. Gsk plc announced today that the us food and drug administration (fda) has granted fast track designation for bepirovirsen, an investigational antisense oligonucleotide. Hepatitis B Cure News.
From egastroenterology.bmj.com
Opportunities and challenges for hepatitis B cure eGastroenterology Hepatitis B Cure News Italian researchers have developed a combination of a drug and an antibody that triggers specific immune cells to fight the hepatitis virus, preventing it from damaging. The biggest unmet need for hepatitis b patients is a cure, but there are a number of trials looking to achieve functional cure for the disease. Gsk’s bepirovirsen, which is looking for a functional. Hepatitis B Cure News.
From www.frontiersin.org
Frontiers Current treatment of chronic hepatitis B Clinical aspects and future directions Hepatitis B Cure News The biggest unmet need for hepatitis b patients is a cure, but there are a number of trials looking to achieve functional cure for the disease. Gsk plc announced today that the us food and drug administration (fda) has granted fast track designation for bepirovirsen, an investigational antisense oligonucleotide (aso) for the treatment of chronic hepatitis b (chb). Maintaining momentum. Hepatitis B Cure News.
From www.verywellhealth.com
Treating Hepatitis B With Tenofovir Hepatitis B Cure News The biggest unmet need for hepatitis b patients is a cure, but there are a number of trials looking to achieve functional cure for the disease. The new nih plan defines a hepatitis b “cure” as a sustained loss of a specific protein on the surface of hbv called hepatitis b virus surface antigen — preferably with antibodies against the. Hepatitis B Cure News.
From www.std-gov.org
Hepatitis B Symptoms, Treatment, Causes, What is Hepatitis B Hepatitis B Cure News A functional cure is said to have been achieved if a biomarker called hepatitis b surface antigen (hbsag) is cleared from the blood and remains undetectable after stopping all treatment. The biggest unmet need for hepatitis b patients is a cure, but there are a number of trials looking to achieve functional cure for the disease. Gsk’s bepirovirsen, which is. Hepatitis B Cure News.
From www.nytimes.com
Hepatitis C Deaths in U.S. Rose in 2014, but New Drugs Hold Promise The New York Times Hepatitis B Cure News Italian researchers have developed a combination of a drug and an antibody that triggers specific immune cells to fight the hepatitis virus, preventing it from damaging. Gsk plc announced today that the us food and drug administration (fda) has granted fast track designation for bepirovirsen, an investigational antisense oligonucleotide (aso) for the treatment of chronic hepatitis b (chb). Innovative curative. Hepatitis B Cure News.
From www.usatoday.com
Girl's AIDS remission after stopping antiHIV drugs encourages scientists Hepatitis B Cure News Gsk plc announced today that the us food and drug administration (fda) has granted fast track designation for bepirovirsen, an investigational antisense oligonucleotide (aso) for the treatment of chronic hepatitis b (chb). Italian researchers have developed a combination of a drug and an antibody that triggers specific immune cells to fight the hepatitis virus, preventing it from damaging. Maintaining momentum. Hepatitis B Cure News.
From www.hepb.org
Hepatitis B Research Review May Hepatitis B Foundation Hepatitis B Cure News Gsk’s bepirovirsen, which is looking for a functional cure, showed very promising results during a phase ii readout in june 2022 and has now initiated a phase iii trial due to end in 2025. A functional cure is said to have been achieved if a biomarker called hepatitis b surface antigen (hbsag) is cleared from the blood and remains undetectable. Hepatitis B Cure News.
From www.usatoday.com
Help with the high cost of hepatitis C drugs Hepatitis B Cure News The new nih plan defines a hepatitis b “cure” as a sustained loss of a specific protein on the surface of hbv called hepatitis b virus surface antigen — preferably with antibodies against the antigen and undetectable viral dna after completion of a finite course of treatment. Italian researchers have developed a combination of a drug and an antibody that. Hepatitis B Cure News.
From www.mdpi.com
Pharmaceuticals Free FullText Recent Advances in Hepatitis B Treatment Hepatitis B Cure News Italian researchers have developed a combination of a drug and an antibody that triggers specific immune cells to fight the hepatitis virus, preventing it from damaging. Gsk plc announced today that the us food and drug administration (fda) has granted fast track designation for bepirovirsen, an investigational antisense oligonucleotide (aso) for the treatment of chronic hepatitis b (chb). Maintaining momentum. Hepatitis B Cure News.
From pursuit.unimelb.edu.au
Exposing the hepatitis B virus Pursuit by The University of Melbourne Hepatitis B Cure News Maintaining momentum in developing such strategies will require continued investment. Innovative curative strategies could reshape the treatment of chronic hepatitis b. The biggest unmet need for hepatitis b patients is a cure, but there are a number of trials looking to achieve functional cure for the disease. Italian researchers have developed a combination of a drug and an antibody that. Hepatitis B Cure News.
From www.mdpi.com
Hepatitis B Virus Cure Targets and Future Therapies Hepatitis B Cure News A functional cure is said to have been achieved if a biomarker called hepatitis b surface antigen (hbsag) is cleared from the blood and remains undetectable after stopping all treatment. Innovative curative strategies could reshape the treatment of chronic hepatitis b. Gsk plc announced today that the us food and drug administration (fda) has granted fast track designation for bepirovirsen,. Hepatitis B Cure News.
From www.usatoday.com
Hepatitis C treatment shifts as new drugs emerge Hepatitis B Cure News Gsk’s bepirovirsen, which is looking for a functional cure, showed very promising results during a phase ii readout in june 2022 and has now initiated a phase iii trial due to end in 2025. Italian researchers have developed a combination of a drug and an antibody that triggers specific immune cells to fight the hepatitis virus, preventing it from damaging.. Hepatitis B Cure News.
From www.thelancet.com
A global scientific strategy to cure hepatitis B The Lancet Gastroenterology & Hepatology Hepatitis B Cure News Gsk’s bepirovirsen, which is looking for a functional cure, showed very promising results during a phase ii readout in june 2022 and has now initiated a phase iii trial due to end in 2025. Maintaining momentum in developing such strategies will require continued investment. The new nih plan defines a hepatitis b “cure” as a sustained loss of a specific. Hepatitis B Cure News.
From www.scmp.com
Hepatitis B cure research health organisations, patient groups, scientists launch campaign Hepatitis B Cure News Italian researchers have developed a combination of a drug and an antibody that triggers specific immune cells to fight the hepatitis virus, preventing it from damaging. Innovative curative strategies could reshape the treatment of chronic hepatitis b. Maintaining momentum in developing such strategies will require continued investment. Gsk plc announced today that the us food and drug administration (fda) has. Hepatitis B Cure News.
From www.mdpi.com
JCM Free FullText New Approaches to the Treatment of Chronic Hepatitis B Hepatitis B Cure News Innovative curative strategies could reshape the treatment of chronic hepatitis b. The new nih plan defines a hepatitis b “cure” as a sustained loss of a specific protein on the surface of hbv called hepatitis b virus surface antigen — preferably with antibodies against the antigen and undetectable viral dna after completion of a finite course of treatment. Maintaining momentum. Hepatitis B Cure News.
From londongastrocare.com
Navigating Hepatitis B Treatment Strategies for Patient Wellbeing London Gastro Care Hepatitis B Cure News The new nih plan defines a hepatitis b “cure” as a sustained loss of a specific protein on the surface of hbv called hepatitis b virus surface antigen — preferably with antibodies against the antigen and undetectable viral dna after completion of a finite course of treatment. Gsk’s bepirovirsen, which is looking for a functional cure, showed very promising results. Hepatitis B Cure News.
From medicalxpress.com
Promising investigational therapeutic monoclonal antibody to treat chronic hepatitis B and D Hepatitis B Cure News The biggest unmet need for hepatitis b patients is a cure, but there are a number of trials looking to achieve functional cure for the disease. The new nih plan defines a hepatitis b “cure” as a sustained loss of a specific protein on the surface of hbv called hepatitis b virus surface antigen — preferably with antibodies against the. Hepatitis B Cure News.
From hepatitiseducation.med.ubc.ca
Hepatitis Education Canada Programme canadien d’éducation sur l’hépatite Hepatitis B Cure News Italian researchers have developed a combination of a drug and an antibody that triggers specific immune cells to fight the hepatitis virus, preventing it from damaging. The new nih plan defines a hepatitis b “cure” as a sustained loss of a specific protein on the surface of hbv called hepatitis b virus surface antigen — preferably with antibodies against the. Hepatitis B Cure News.