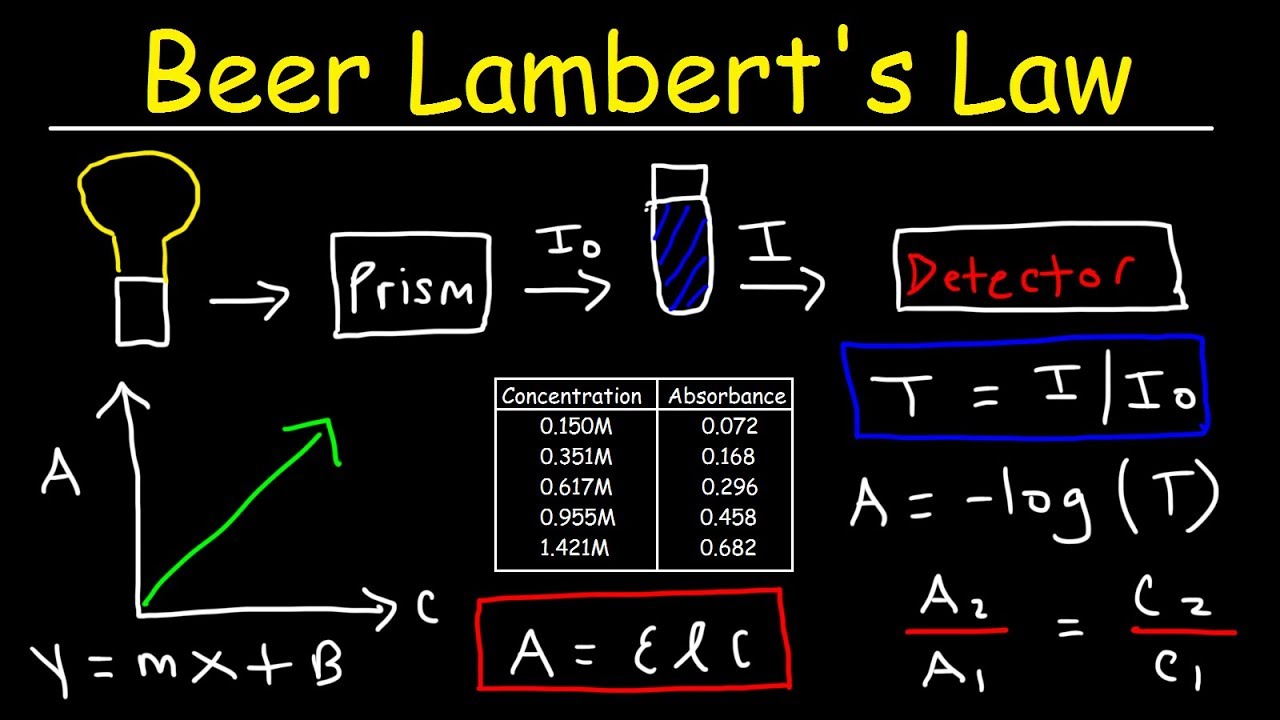
Sources Of Error In Beer's Law Lab . Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Application of beer's law to analyses. The simplest relies on measuring the absorbance of a known sample. The direct relationship between absorbance and concentration for a solution is known as beer’s law. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The concentration of an unknown niso4. Beer’s law fabrizio chigne valerga lab partner: The beer's law equation may be applied to analyses in a variety of ways. Determine order of reaction by measuring the concentration of. This relationship is called the. The primary objective of this experiment is to determine the concentration of a common food dye, allura red, in various red.
from www.youtube.com
In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer’s law fabrizio chigne valerga lab partner: The simplest relies on measuring the absorbance of a known sample. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. The direct relationship between absorbance and concentration for a solution is known as beer’s law. The primary objective of this experiment is to determine the concentration of a common food dye, allura red, in various red. The concentration of an unknown niso4. Application of beer's law to analyses. Determine order of reaction by measuring the concentration of. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law).
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry, Basic Introduction
Sources Of Error In Beer's Law Lab The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Application of beer's law to analyses. The simplest relies on measuring the absorbance of a known sample. The primary objective of this experiment is to determine the concentration of a common food dye, allura red, in various red. This relationship is called the. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer’s law fabrizio chigne valerga lab partner: Determine order of reaction by measuring the concentration of. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). The concentration of an unknown niso4. The beer's law equation may be applied to analyses in a variety of ways. The direct relationship between absorbance and concentration for a solution is known as beer’s law.
From studylib.net
Beer's Law Lab Sources Of Error In Beer's Law Lab The beer's law equation may be applied to analyses in a variety of ways. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In most experiments, molar absorptivity (ε) and the. Sources Of Error In Beer's Law Lab.
From www.scribd.com
Beer S Law Lab Instructions PDF Absorbance Molar Concentration Sources Of Error In Beer's Law Lab In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The concentration of an unknown niso4. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. The beer's law equation may be applied to analyses in a variety of ways. The simplest. Sources Of Error In Beer's Law Lab.
From sciencenotes.org
Beer's Law Equation and Example Sources Of Error In Beer's Law Lab The concentration of an unknown niso4. The direct relationship between absorbance and concentration for a solution is known as beer’s law. The primary objective of this experiment is to determine the concentration of a common food dye, allura red, in various red. The beer's law equation may be applied to analyses in a variety of ways. The simplest relies on. Sources Of Error In Beer's Law Lab.
From goformative.com
Beer's Law Simulation for AP Chemistry Carson Dobrin Library Formative Sources Of Error In Beer's Law Lab Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). The primary objective of this experiment is to determine the concentration of a common food dye, allura red, in various red. The direct relationship between absorbance and concentration for a solution is known as beer’s law. The beer's law equation may be applied to analyses in a. Sources Of Error In Beer's Law Lab.
From www.vernier.com
Beer's Law Investigations > Experiment 11 from Investigating Chemistry through Inquiry Sources Of Error In Beer's Law Lab Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). The primary objective of this experiment is to determine the concentration of a common food dye, allura red, in various red. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. The concentration of an. Sources Of Error In Beer's Law Lab.
From exotdewyh.blob.core.windows.net
Beer's Law Lab Sources Of Error at Lisa Doyle blog Sources Of Error In Beer's Law Lab Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The beer's law equation may be applied to analyses in a variety of ways. Application of beer's law to. Sources Of Error In Beer's Law Lab.
From studylib.net
Beer's Law Lab Sources Of Error In Beer's Law Lab The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. This relationship is called the. Determine order of reaction by measuring the concentration. Sources Of Error In Beer's Law Lab.
From www.thuvienstem.edu.vn
Beer's Law Lab Thư viện Stem Sources Of Error In Beer's Law Lab The beer's law equation may be applied to analyses in a variety of ways. The simplest relies on measuring the absorbance of a known sample. The direct relationship between absorbance and concentration for a solution is known as beer’s law. The primary objective of this experiment is to determine the concentration of a common food dye, allura red, in various. Sources Of Error In Beer's Law Lab.
From studylib.net
Lab 7 Beer's Law Sources Of Error In Beer's Law Lab The simplest relies on measuring the absorbance of a known sample. The direct relationship between absorbance and concentration for a solution is known as beer’s law. Determine order of reaction by measuring the concentration of. This relationship is called the. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Since the concentration, path. Sources Of Error In Beer's Law Lab.
From www.youtube.com
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry, Basic Introduction Sources Of Error In Beer's Law Lab Beer’s law fabrizio chigne valerga lab partner: The direct relationship between absorbance and concentration for a solution is known as beer’s law. The primary objective of this experiment is to determine the concentration of a common food dye, allura red, in various red. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The. Sources Of Error In Beer's Law Lab.
From www.slideserve.com
PPT Chem. 133 3/18 Lecture PowerPoint Presentation, free download ID3597161 Sources Of Error In Beer's Law Lab The simplest relies on measuring the absorbance of a known sample. Determine order of reaction by measuring the concentration of. Application of beer's law to analyses. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). The beer's law equation may be applied to analyses in a variety of ways. Beer’s law fabrizio chigne valerga lab partner:. Sources Of Error In Beer's Law Lab.
From www.studocu.com
Beer's Law Lab Beer's Law, 20192020 academic year Experiment 4 Beer’s Law. Purpose The Sources Of Error In Beer's Law Lab Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Application of beer's law to analyses. This relationship is called the. Beer’s law fabrizio chigne valerga lab partner: The simplest relies on measuring the absorbance of a known sample. The primary objective of this experiment is to determine the concentration of a common food dye, allura red,. Sources Of Error In Beer's Law Lab.
From studylib.net
Lab 4 Determination of Solution Concentration Using Beer's Law Sources Of Error In Beer's Law Lab The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. This relationship is called the. Beer’s law fabrizio chigne valerga lab partner: In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Determine order of reaction by measuring the concentration of. The. Sources Of Error In Beer's Law Lab.
From www.studocu.com
Lab report Experiment 2. Spectroscopic Analysis using Beer’s Law. Experiment 2. Name Sources Of Error In Beer's Law Lab In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The beer's law equation may be applied to analyses in a variety of ways. The concentration of an unknown niso4. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known. Sources Of Error In Beer's Law Lab.
From studylib.net
Beer's Law Lab Sources Of Error In Beer's Law Lab The direct relationship between absorbance and concentration for a solution is known as beer’s law. This relationship is called the. The simplest relies on measuring the absorbance of a known sample. The beer's law equation may be applied to analyses in a variety of ways. The amount of light that a species absorbs in a spectroscopic transition can be related. Sources Of Error In Beer's Law Lab.
From www.youtube.com
BeerLambert law in easy way YouTube Sources Of Error In Beer's Law Lab The primary objective of this experiment is to determine the concentration of a common food dye, allura red, in various red. Beer’s law fabrizio chigne valerga lab partner: The simplest relies on measuring the absorbance of a known sample. The concentration of an unknown niso4. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance,. Sources Of Error In Beer's Law Lab.
From www.chegg.com
Solved SPECTROPHOTOMETRY. APPLY BEER'S LAW TO DETERMINE Sources Of Error In Beer's Law Lab Beer’s law fabrizio chigne valerga lab partner: The beer's law equation may be applied to analyses in a variety of ways. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. The simplest relies on measuring the absorbance of a known sample. This relationship is called the. The. Sources Of Error In Beer's Law Lab.
From www.researchgate.net
Errors in dye concentration prediction by Beer's law at 3 wavelengths. Download Scientific Diagram Sources Of Error In Beer's Law Lab Application of beer's law to analyses. The concentration of an unknown niso4. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The direct relationship between absorbance and concentration for a solution is known as beer’s law. The beer's law equation may be applied to analyses in a variety of ways. Calculate the concentration. Sources Of Error In Beer's Law Lab.
From www.youtube.com
Ch 17 8 Error in Beer's Law YouTube Sources Of Error In Beer's Law Lab The direct relationship between absorbance and concentration for a solution is known as beer’s law. Application of beer's law to analyses. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. This relationship is called the. The concentration of an unknown niso4. Beer’s law fabrizio chigne valerga lab. Sources Of Error In Beer's Law Lab.
From exotdewyh.blob.core.windows.net
Beer's Law Lab Sources Of Error at Lisa Doyle blog Sources Of Error In Beer's Law Lab The direct relationship between absorbance and concentration for a solution is known as beer’s law. The simplest relies on measuring the absorbance of a known sample. Determine order of reaction by measuring the concentration of. This relationship is called the. Application of beer's law to analyses. The primary objective of this experiment is to determine the concentration of a common. Sources Of Error In Beer's Law Lab.
From www.thoughtco.com
Beer's Law Definition and Equation Sources Of Error In Beer's Law Lab The simplest relies on measuring the absorbance of a known sample. The direct relationship between absorbance and concentration for a solution is known as beer’s law. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the. Sources Of Error In Beer's Law Lab.
From www.youtube.com
Beer's Law Overview YouTube Sources Of Error In Beer's Law Lab The simplest relies on measuring the absorbance of a known sample. Beer’s law fabrizio chigne valerga lab partner: The beer's law equation may be applied to analyses in a variety of ways. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The concentration of. Sources Of Error In Beer's Law Lab.
From www.wam.umd.edu
Simulation of Instrumental Deviation from Beer's Law Sources Of Error In Beer's Law Lab This relationship is called the. Determine order of reaction by measuring the concentration of. The concentration of an unknown niso4. Application of beer's law to analyses. The primary objective of this experiment is to determine the concentration of a common food dye, allura red, in various red. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law).. Sources Of Error In Beer's Law Lab.
From studylib.net
Beer`s Law Lab Sources Of Error In Beer's Law Lab Determine order of reaction by measuring the concentration of. The beer's law equation may be applied to analyses in a variety of ways. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). The direct. Sources Of Error In Beer's Law Lab.
From studylib.net
Beer's Law Lab Sources Of Error In Beer's Law Lab The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Application of beer's law to analyses. The direct relationship between absorbance and concentration for a solution is known as beer’s law. The. Sources Of Error In Beer's Law Lab.
From exotdewyh.blob.core.windows.net
Beer's Law Lab Sources Of Error at Lisa Doyle blog Sources Of Error In Beer's Law Lab The beer's law equation may be applied to analyses in a variety of ways. The simplest relies on measuring the absorbance of a known sample. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The direct relationship between absorbance and concentration for a solution is known as beer’s law. Since the concentration, path. Sources Of Error In Beer's Law Lab.
From ohmlaw.com
5 Error Sources in Ohm's Law Experiment [How to avoid them] • Ohm Law Sources Of Error In Beer's Law Lab The beer's law equation may be applied to analyses in a variety of ways. The direct relationship between absorbance and concentration for a solution is known as beer’s law. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The primary objective of this experiment. Sources Of Error In Beer's Law Lab.
From exotdewyh.blob.core.windows.net
Beer's Law Lab Sources Of Error at Lisa Doyle blog Sources Of Error In Beer's Law Lab This relationship is called the. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The concentration of an unknown niso4. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. The direct. Sources Of Error In Beer's Law Lab.
From www.youtube.com
Spectrophotometry and Beer's Law YouTube Sources Of Error In Beer's Law Lab Application of beer's law to analyses. The concentration of an unknown niso4. The direct relationship between absorbance and concentration for a solution is known as beer’s law. The simplest relies on measuring the absorbance of a known sample. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species.. Sources Of Error In Beer's Law Lab.
From www.youtube.com
Experiment 1 Verify LambertBeer's Law. YouTube Sources Of Error In Beer's Law Lab The simplest relies on measuring the absorbance of a known sample. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The concentration of an unknown niso4. The primary objective of this experiment is to determine the concentration of a common food dye, allura red,. Sources Of Error In Beer's Law Lab.
From calculatorghw.blogspot.com
Beer Lambert Law Calculator CALCULATOR GHW Sources Of Error In Beer's Law Lab Beer’s law fabrizio chigne valerga lab partner: The primary objective of this experiment is to determine the concentration of a common food dye, allura red, in various red. This relationship is called the. The beer's law equation may be applied to analyses in a variety of ways. Determine order of reaction by measuring the concentration of. Since the concentration, path. Sources Of Error In Beer's Law Lab.
From www.studocu.com
Lab 4 Beer's Law Lab 4 Beer’s Law OBJECTIVES. The purpose of this experiment is to Sources Of Error In Beer's Law Lab Application of beer's law to analyses. Determine order of reaction by measuring the concentration of. The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species. The beer's law equation may be applied to analyses in a variety of ways. Calculate the concentration of a dilute aqueous solute using. Sources Of Error In Beer's Law Lab.
From www.studocu.com
Beer's Law Experiment Experiment 4 Beer’s Law. Purpose The purpose of this lab is to Sources Of Error In Beer's Law Lab Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The beer's law equation may be applied to analyses in a variety of ways. This relationship is called the.. Sources Of Error In Beer's Law Lab.
From www.youtube.com
Beer Lambert law derivation and usage YouTube Sources Of Error In Beer's Law Lab Calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). The simplest relies on measuring the absorbance of a known sample. The primary objective of this experiment is to determine the concentration of a common food dye, allura red, in various red. The amount of light that a species absorbs in a spectroscopic transition can be related. Sources Of Error In Beer's Law Lab.
From www.softpedia.com
Download Beer's Law Lab Sources Of Error In Beer's Law Lab Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The primary objective of this experiment is to determine the concentration of a common food dye, allura red, in various red. Application of beer's law to analyses. Calculate the concentration of a dilute aqueous solute. Sources Of Error In Beer's Law Lab.