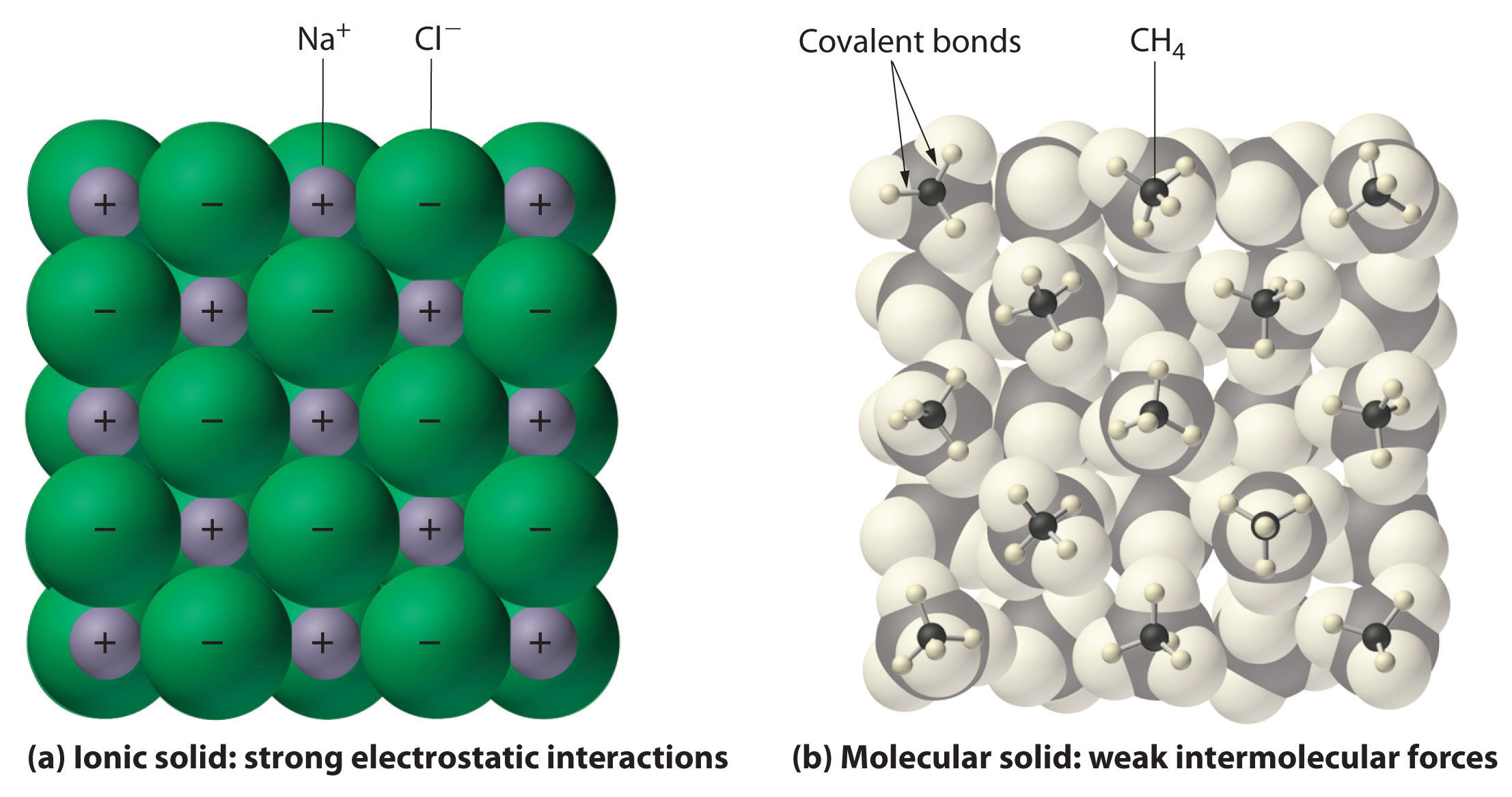
How Do Chemists Model Ionic Compounds . Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Shiny element that is a good conductor of electricity and heat, and which forms. an ionic compound is made up of two or more ions (charged particles) which are held together by electrostatic attraction. forming ionic bonds. An ionic bond is the strongest type of chemical bond,. ionic compounds form when atoms connect to one another by ionic bonds. ionic compounds form hard crystalline solids that melt at high temperatures and are resistant to evaporation. covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of. Positive and negative ions form when a. it is founded on the idea that a pair of electrons shared between two atoms can create a mutual attraction, and thus a chemical bond.
from chem.libretexts.org
Positive and negative ions form when a. ionic compounds form hard crystalline solids that melt at high temperatures and are resistant to evaporation. Shiny element that is a good conductor of electricity and heat, and which forms. ionic compounds form when atoms connect to one another by ionic bonds. An ionic bond is the strongest type of chemical bond,. it is founded on the idea that a pair of electrons shared between two atoms can create a mutual attraction, and thus a chemical bond. an ionic compound is made up of two or more ions (charged particles) which are held together by electrostatic attraction. forming ionic bonds. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of.
3.1 Types of Chemical Compounds and their Formulas Chemistry LibreTexts
How Do Chemists Model Ionic Compounds Positive and negative ions form when a. Shiny element that is a good conductor of electricity and heat, and which forms. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Positive and negative ions form when a. An ionic bond is the strongest type of chemical bond,. ionic compounds form when atoms connect to one another by ionic bonds. ionic compounds form hard crystalline solids that melt at high temperatures and are resistant to evaporation. forming ionic bonds. an ionic compound is made up of two or more ions (charged particles) which are held together by electrostatic attraction. it is founded on the idea that a pair of electrons shared between two atoms can create a mutual attraction, and thus a chemical bond. covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of.
From www.studypool.com
SOLUTION Chemical formulas of ionic compounds Studypool How Do Chemists Model Ionic Compounds Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of. an ionic compound is made up of two or more ions (charged particles) which are held together by electrostatic. How Do Chemists Model Ionic Compounds.
From ec.bertabulle.com
How Do Ions Conduct Electricity How Do Chemists Model Ionic Compounds covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of. it is founded on the idea that a pair of electrons shared between two atoms can create a mutual attraction, and thus a chemical bond. Positive and negative ions form when a. Shiny element that is a. How Do Chemists Model Ionic Compounds.
From slideplayer.com
Aim How do chemists classify types of chemical reactions? ppt download How Do Chemists Model Ionic Compounds ionic compounds form when atoms connect to one another by ionic bonds. ionic compounds form hard crystalline solids that melt at high temperatures and are resistant to evaporation. Positive and negative ions form when a. Shiny element that is a good conductor of electricity and heat, and which forms. Ionic compounds have high melting and boiling points, so. How Do Chemists Model Ionic Compounds.
From www.thoughtco.com
Examples of Ionic Bonds and Compounds How Do Chemists Model Ionic Compounds ionic compounds form hard crystalline solids that melt at high temperatures and are resistant to evaporation. An ionic bond is the strongest type of chemical bond,. Shiny element that is a good conductor of electricity and heat, and which forms. ionic compounds form when atoms connect to one another by ionic bonds. Positive and negative ions form when. How Do Chemists Model Ionic Compounds.
From studylib.net
III. Ionic Compounds How Do Chemists Model Ionic Compounds ionic compounds form hard crystalline solids that melt at high temperatures and are resistant to evaporation. Shiny element that is a good conductor of electricity and heat, and which forms. An ionic bond is the strongest type of chemical bond,. covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or. How Do Chemists Model Ionic Compounds.
From www.learnatnoon.com
Physical Properties of an Ionic Compound Noon Academy How Do Chemists Model Ionic Compounds Positive and negative ions form when a. forming ionic bonds. ionic compounds form when atoms connect to one another by ionic bonds. ionic compounds form hard crystalline solids that melt at high temperatures and are resistant to evaporation. it is founded on the idea that a pair of electrons shared between two atoms can create a. How Do Chemists Model Ionic Compounds.
From www.youtube.com
Ionic Bonds & Compounds in Chemistry [1218] YouTube How Do Chemists Model Ionic Compounds Positive and negative ions form when a. an ionic compound is made up of two or more ions (charged particles) which are held together by electrostatic attraction. covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of. Shiny element that is a good conductor of electricity and. How Do Chemists Model Ionic Compounds.
From www.studocu.com
Ionic Compounds Student Note + Worksheet C09 Name How Do Chemists Model Ionic Compounds An ionic bond is the strongest type of chemical bond,. ionic compounds form hard crystalline solids that melt at high temperatures and are resistant to evaporation. forming ionic bonds. Shiny element that is a good conductor of electricity and heat, and which forms. Positive and negative ions form when a. it is founded on the idea that. How Do Chemists Model Ionic Compounds.
From utaheducationfacts.com
How To Write Ionic Compounds How Do Chemists Model Ionic Compounds Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. forming ionic bonds. ionic compounds form when atoms connect to one another by ionic bonds. An ionic bond is the strongest type of chemical bond,. Shiny element that is a good conductor of electricity and heat, and which forms. . How Do Chemists Model Ionic Compounds.
From www.thoughtco.com
Examples of Ionic Bonds and Compounds How Do Chemists Model Ionic Compounds Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. it is founded on the idea that a pair of electrons shared between two atoms can create a mutual attraction, and thus a chemical bond. ionic compounds form when atoms connect to one another by ionic bonds. forming ionic. How Do Chemists Model Ionic Compounds.
From www.animalia-life.club
Properties Of Ionic Compounds How Do Chemists Model Ionic Compounds Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Positive and negative ions form when a. ionic compounds form hard crystalline solids that melt at high temperatures and are resistant to evaporation. forming ionic bonds. it is founded on the idea that a pair of electrons shared between. How Do Chemists Model Ionic Compounds.
From biologydictionary.net
Ionic Bond Examples Biology Dictionary How Do Chemists Model Ionic Compounds covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. it is founded on the idea that a pair of electrons shared between two atoms can create a mutual. How Do Chemists Model Ionic Compounds.
From www.thoughtco.com
Why the Formation of Ionic Compounds Is Exothermic How Do Chemists Model Ionic Compounds Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Shiny element that is a good conductor of electricity and heat, and which forms. Positive and negative ions form when a. ionic compounds form hard crystalline solids that melt at high temperatures and are resistant to evaporation. forming ionic bonds.. How Do Chemists Model Ionic Compounds.
From www.breakingatom.com
Ionic Properties How Do Chemists Model Ionic Compounds Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Positive and negative ions form when a. an ionic compound is made up of two or more ions (charged particles) which are held together by electrostatic attraction. covalent bonds are the attractive forces between the positively charged nuclei of the. How Do Chemists Model Ionic Compounds.
From www.pinterest.com
Pin by ChemKate Chemistry lessons, on Nomenclature and Ions Ionic How Do Chemists Model Ionic Compounds ionic compounds form hard crystalline solids that melt at high temperatures and are resistant to evaporation. an ionic compound is made up of two or more ions (charged particles) which are held together by electrostatic attraction. Shiny element that is a good conductor of electricity and heat, and which forms. forming ionic bonds. Positive and negative ions. How Do Chemists Model Ionic Compounds.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts How Do Chemists Model Ionic Compounds ionic compounds form hard crystalline solids that melt at high temperatures and are resistant to evaporation. forming ionic bonds. Shiny element that is a good conductor of electricity and heat, and which forms. covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of. An ionic bond. How Do Chemists Model Ionic Compounds.
From slideplayer.com
Ionic Compounds Chapter ppt download How Do Chemists Model Ionic Compounds ionic compounds form hard crystalline solids that melt at high temperatures and are resistant to evaporation. covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of. ionic compounds form when atoms connect to one another by ionic bonds. an ionic compound is made up of. How Do Chemists Model Ionic Compounds.
From slideplayer.com
Aim How do chemists write and interpret chemical formulas? ppt download How Do Chemists Model Ionic Compounds An ionic bond is the strongest type of chemical bond,. Shiny element that is a good conductor of electricity and heat, and which forms. an ionic compound is made up of two or more ions (charged particles) which are held together by electrostatic attraction. forming ionic bonds. covalent bonds are the attractive forces between the positively charged. How Do Chemists Model Ionic Compounds.
From chem.libretexts.org
3.1 Types of Chemical Compounds and their Formulas Chemistry LibreTexts How Do Chemists Model Ionic Compounds ionic compounds form when atoms connect to one another by ionic bonds. covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of. Positive and negative ions form when a. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature.. How Do Chemists Model Ionic Compounds.
From www.jagranjosh.com
What are Ionic Compounds and how they are formed? How Do Chemists Model Ionic Compounds forming ionic bonds. ionic compounds form when atoms connect to one another by ionic bonds. an ionic compound is made up of two or more ions (charged particles) which are held together by electrostatic attraction. it is founded on the idea that a pair of electrons shared between two atoms can create a mutual attraction, and. How Do Chemists Model Ionic Compounds.
From slidetodoc.com
Aim How do chemists distinguish between ionic covalent How Do Chemists Model Ionic Compounds An ionic bond is the strongest type of chemical bond,. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. it is founded on the idea that a pair of electrons shared between two atoms can create a mutual attraction, and thus a chemical bond. forming ionic bonds. Shiny element. How Do Chemists Model Ionic Compounds.
From imsyaf.com
Naming Ionic Compounds Worksheet Answers How Do Chemists Model Ionic Compounds ionic compounds form when atoms connect to one another by ionic bonds. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. it is founded on the idea that a pair of electrons shared between two atoms can create a mutual attraction, and thus a chemical bond. An ionic bond. How Do Chemists Model Ionic Compounds.
From users.highland.edu
Naming Ionic Compounds How Do Chemists Model Ionic Compounds An ionic bond is the strongest type of chemical bond,. Positive and negative ions form when a. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. ionic compounds form when atoms connect to one another by ionic bonds. it is founded on the idea that a pair of electrons. How Do Chemists Model Ionic Compounds.
From dokumen.tips
(PPT) Ionic Compounds Ionic Compounds. Chemistry Joke Q Why do How Do Chemists Model Ionic Compounds ionic compounds form hard crystalline solids that melt at high temperatures and are resistant to evaporation. it is founded on the idea that a pair of electrons shared between two atoms can create a mutual attraction, and thus a chemical bond. Positive and negative ions form when a. covalent bonds are the attractive forces between the positively. How Do Chemists Model Ionic Compounds.
From slideplayer.com
Aim How do chemists classify types of chemical reactions? ppt download How Do Chemists Model Ionic Compounds an ionic compound is made up of two or more ions (charged particles) which are held together by electrostatic attraction. An ionic bond is the strongest type of chemical bond,. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. covalent bonds are the attractive forces between the positively charged. How Do Chemists Model Ionic Compounds.
From sciencenotes.org
What Is a Compound in Chemistry? Definition and Examples How Do Chemists Model Ionic Compounds Shiny element that is a good conductor of electricity and heat, and which forms. ionic compounds form when atoms connect to one another by ionic bonds. Positive and negative ions form when a. ionic compounds form hard crystalline solids that melt at high temperatures and are resistant to evaporation. forming ionic bonds. covalent bonds are the. How Do Chemists Model Ionic Compounds.
From www.shalom-education.com
Properties of Ionic Compounds GCSE Chemistry Revision How Do Chemists Model Ionic Compounds an ionic compound is made up of two or more ions (charged particles) which are held together by electrostatic attraction. covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of. Positive and negative ions form when a. Shiny element that is a good conductor of electricity and. How Do Chemists Model Ionic Compounds.
From www.toppr.com
Ionic Compounds Ionic bonds, Properties, Formation, Examples, Videos How Do Chemists Model Ionic Compounds ionic compounds form when atoms connect to one another by ionic bonds. it is founded on the idea that a pair of electrons shared between two atoms can create a mutual attraction, and thus a chemical bond. An ionic bond is the strongest type of chemical bond,. forming ionic bonds. Positive and negative ions form when a.. How Do Chemists Model Ionic Compounds.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures How Do Chemists Model Ionic Compounds ionic compounds form hard crystalline solids that melt at high temperatures and are resistant to evaporation. An ionic bond is the strongest type of chemical bond,. forming ionic bonds. an ionic compound is made up of two or more ions (charged particles) which are held together by electrostatic attraction. ionic compounds form when atoms connect to. How Do Chemists Model Ionic Compounds.
From quizizz.com
Properties of ionic compounds Chemistry Quizizz How Do Chemists Model Ionic Compounds covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of. an ionic compound is made up of two or more ions (charged particles) which are held together by electrostatic attraction. forming ionic bonds. Positive and negative ions form when a. Shiny element that is a good. How Do Chemists Model Ionic Compounds.
From www.sliderbase.com
Ionic Bonding Presentation Chemistry How Do Chemists Model Ionic Compounds Shiny element that is a good conductor of electricity and heat, and which forms. Positive and negative ions form when a. An ionic bond is the strongest type of chemical bond,. ionic compounds form hard crystalline solids that melt at high temperatures and are resistant to evaporation. covalent bonds are the attractive forces between the positively charged nuclei. How Do Chemists Model Ionic Compounds.
From www.youtube.com
How To Draw The Lewis Structures of Ionic Compounds YouTube How Do Chemists Model Ionic Compounds Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Positive and negative ions form when a. covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of. Shiny element that is a good conductor of electricity and heat, and which. How Do Chemists Model Ionic Compounds.
From slidetodoc.com
Aim How do chemists distinguish between ionic covalent How Do Chemists Model Ionic Compounds Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. forming ionic bonds. ionic compounds form when atoms connect to one another by ionic bonds. ionic compounds form hard crystalline solids that melt at high temperatures and are resistant to evaporation. Shiny element that is a good conductor of. How Do Chemists Model Ionic Compounds.
From www.youtube.com
How To Name Ionic Compounds In Chemistry YouTube How Do Chemists Model Ionic Compounds Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. forming ionic bonds. it is founded on the idea that a pair of electrons shared between two atoms can create a mutual attraction, and thus a chemical bond. covalent bonds are the attractive forces between the positively charged nuclei. How Do Chemists Model Ionic Compounds.
From shareeducatonideas.com
What Is An Ionic Compound? Formula and Defination How Do Chemists Model Ionic Compounds Shiny element that is a good conductor of electricity and heat, and which forms. covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of. An ionic bond is the strongest type of chemical bond,. it is founded on the idea that a pair of electrons shared between. How Do Chemists Model Ionic Compounds.