Tungsten Oxidation Number . When present in compounds, tungsten exists mostly in the oxidation state vi. Tungsten is a chemical element with atomic number 74 which means there are 74 protons and 74 electrons in the atomic. It is defined as being the charge that. A positive or negative integer that represents the number of electrons that an atom has gained or lost in a chemical compound. The oxidation state of an atom is a measure of the degree of oxidation of an atom. The most common and stable state is +6, seen in compounds like tungsten trioxide (wo 3). Higher oxidation numbers are relevant to its terrestrial occurrence and its biological roles. Tungsten exhibits a wide range of oxidation numbers from 0 to +6. Tungsten and its alloys are widely used for filaments in older style (not energy saving).
from www.semanticscholar.org
Tungsten exhibits a wide range of oxidation numbers from 0 to +6. Higher oxidation numbers are relevant to its terrestrial occurrence and its biological roles. A positive or negative integer that represents the number of electrons that an atom has gained or lost in a chemical compound. The oxidation state of an atom is a measure of the degree of oxidation of an atom. Tungsten is a chemical element with atomic number 74 which means there are 74 protons and 74 electrons in the atomic. When present in compounds, tungsten exists mostly in the oxidation state vi. It is defined as being the charge that. Tungsten and its alloys are widely used for filaments in older style (not energy saving). The most common and stable state is +6, seen in compounds like tungsten trioxide (wo 3).
Figure 2.1 from Thermodynamics and of Tungsten Oxidation and
Tungsten Oxidation Number The oxidation state of an atom is a measure of the degree of oxidation of an atom. Tungsten and its alloys are widely used for filaments in older style (not energy saving). The oxidation state of an atom is a measure of the degree of oxidation of an atom. When present in compounds, tungsten exists mostly in the oxidation state vi. It is defined as being the charge that. Tungsten exhibits a wide range of oxidation numbers from 0 to +6. Higher oxidation numbers are relevant to its terrestrial occurrence and its biological roles. A positive or negative integer that represents the number of electrons that an atom has gained or lost in a chemical compound. Tungsten is a chemical element with atomic number 74 which means there are 74 protons and 74 electrons in the atomic. The most common and stable state is +6, seen in compounds like tungsten trioxide (wo 3).
From www.researchgate.net
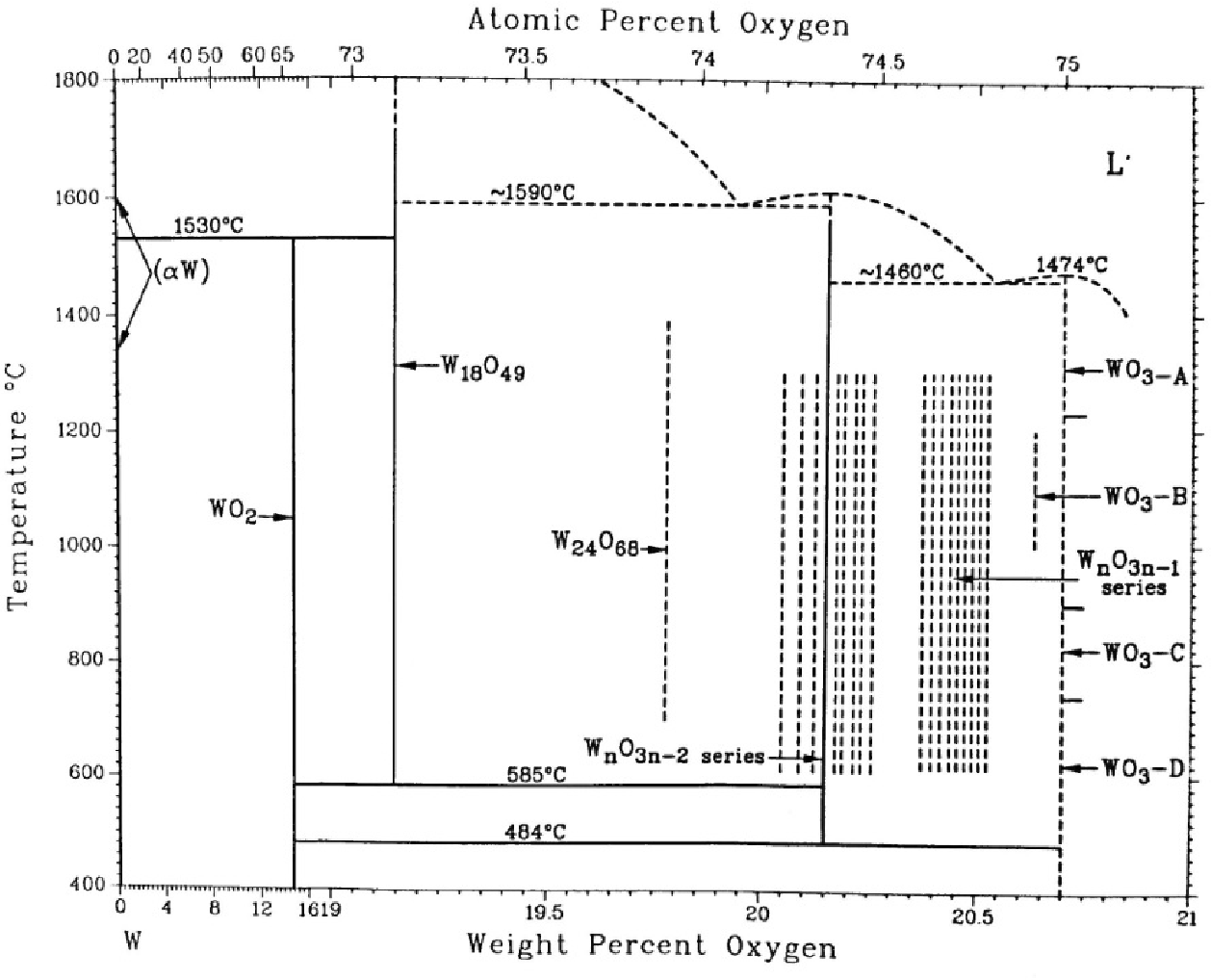
Oxygentungsten phase diagram (redrawn from Ref. [36] and extended down Tungsten Oxidation Number Tungsten is a chemical element with atomic number 74 which means there are 74 protons and 74 electrons in the atomic. Higher oxidation numbers are relevant to its terrestrial occurrence and its biological roles. The most common and stable state is +6, seen in compounds like tungsten trioxide (wo 3). Tungsten and its alloys are widely used for filaments in. Tungsten Oxidation Number.
From shiken.ai
Oxidation Number Tungsten Oxidation Number Tungsten exhibits a wide range of oxidation numbers from 0 to +6. The most common and stable state is +6, seen in compounds like tungsten trioxide (wo 3). The oxidation state of an atom is a measure of the degree of oxidation of an atom. It is defined as being the charge that. Tungsten is a chemical element with atomic. Tungsten Oxidation Number.
From www.researchgate.net
Tungsten filament of 50 μm after treatment at (a, b) 250 h which a Tungsten Oxidation Number Tungsten exhibits a wide range of oxidation numbers from 0 to +6. Tungsten and its alloys are widely used for filaments in older style (not energy saving). It is defined as being the charge that. The most common and stable state is +6, seen in compounds like tungsten trioxide (wo 3). A positive or negative integer that represents the number. Tungsten Oxidation Number.
From www.alamy.com
Tungsten (W). Diagram of the nuclear composition, electron Tungsten Oxidation Number Tungsten and its alloys are widely used for filaments in older style (not energy saving). Tungsten is a chemical element with atomic number 74 which means there are 74 protons and 74 electrons in the atomic. It is defined as being the charge that. The most common and stable state is +6, seen in compounds like tungsten trioxide (wo 3).. Tungsten Oxidation Number.
From studylib.net
Oxidation Numbers Tungsten Oxidation Number The most common and stable state is +6, seen in compounds like tungsten trioxide (wo 3). A positive or negative integer that represents the number of electrons that an atom has gained or lost in a chemical compound. Tungsten exhibits a wide range of oxidation numbers from 0 to +6. The oxidation state of an atom is a measure of. Tungsten Oxidation Number.
From onlinelibrary.wiley.com
Review on the Versatility of Tungsten Oxide Coatings Mardare 2019 Tungsten Oxidation Number Higher oxidation numbers are relevant to its terrestrial occurrence and its biological roles. Tungsten exhibits a wide range of oxidation numbers from 0 to +6. A positive or negative integer that represents the number of electrons that an atom has gained or lost in a chemical compound. The most common and stable state is +6, seen in compounds like tungsten. Tungsten Oxidation Number.
From www.nuclear-power.com
Tungsten Atomic Number Atomic Mass Density of Tungsten nuclear Tungsten Oxidation Number When present in compounds, tungsten exists mostly in the oxidation state vi. Tungsten and its alloys are widely used for filaments in older style (not energy saving). A positive or negative integer that represents the number of electrons that an atom has gained or lost in a chemical compound. Tungsten is a chemical element with atomic number 74 which means. Tungsten Oxidation Number.
From www.vecteezy.com
Tungsten symbol. Chemical element of the periodic table. Vector Tungsten Oxidation Number The oxidation state of an atom is a measure of the degree of oxidation of an atom. Tungsten and its alloys are widely used for filaments in older style (not energy saving). The most common and stable state is +6, seen in compounds like tungsten trioxide (wo 3). Higher oxidation numbers are relevant to its terrestrial occurrence and its biological. Tungsten Oxidation Number.
From www.researchgate.net
SEM images of tungsten oxide prepared (A, B) by normal hydrothermal Tungsten Oxidation Number When present in compounds, tungsten exists mostly in the oxidation state vi. The most common and stable state is +6, seen in compounds like tungsten trioxide (wo 3). A positive or negative integer that represents the number of electrons that an atom has gained or lost in a chemical compound. It is defined as being the charge that. Tungsten and. Tungsten Oxidation Number.
From chemistrycommunity.nature.com
Can Single Atom Tungsten Enhance Electrocatalytic Water Oxidation Tungsten Oxidation Number Tungsten exhibits a wide range of oxidation numbers from 0 to +6. The most common and stable state is +6, seen in compounds like tungsten trioxide (wo 3). A positive or negative integer that represents the number of electrons that an atom has gained or lost in a chemical compound. The oxidation state of an atom is a measure of. Tungsten Oxidation Number.
From www.researchgate.net
(a) Tungstenoxygen phase diagram. (b) Detail of tungsten oxygen phase Tungsten Oxidation Number The most common and stable state is +6, seen in compounds like tungsten trioxide (wo 3). When present in compounds, tungsten exists mostly in the oxidation state vi. The oxidation state of an atom is a measure of the degree of oxidation of an atom. A positive or negative integer that represents the number of electrons that an atom has. Tungsten Oxidation Number.
From sciencenotes.org
Downloadable Periodic Table Oxidation States Tungsten Oxidation Number It is defined as being the charge that. The oxidation state of an atom is a measure of the degree of oxidation of an atom. The most common and stable state is +6, seen in compounds like tungsten trioxide (wo 3). A positive or negative integer that represents the number of electrons that an atom has gained or lost in. Tungsten Oxidation Number.
From chem.libretexts.org
Oxidation States of Transition Metals Chemistry LibreTexts Tungsten Oxidation Number Tungsten and its alloys are widely used for filaments in older style (not energy saving). The most common and stable state is +6, seen in compounds like tungsten trioxide (wo 3). Higher oxidation numbers are relevant to its terrestrial occurrence and its biological roles. Tungsten is a chemical element with atomic number 74 which means there are 74 protons and. Tungsten Oxidation Number.
From valenceelectrons.com
Protons, Neutrons, Electrons for Tungsten (W, W6+) Tungsten Oxidation Number The most common and stable state is +6, seen in compounds like tungsten trioxide (wo 3). Tungsten and its alloys are widely used for filaments in older style (not energy saving). Higher oxidation numbers are relevant to its terrestrial occurrence and its biological roles. It is defined as being the charge that. The oxidation state of an atom is a. Tungsten Oxidation Number.
From www.semanticscholar.org
Figure 2.1 from Thermodynamics and of Tungsten Oxidation and Tungsten Oxidation Number When present in compounds, tungsten exists mostly in the oxidation state vi. Tungsten exhibits a wide range of oxidation numbers from 0 to +6. The oxidation state of an atom is a measure of the degree of oxidation of an atom. A positive or negative integer that represents the number of electrons that an atom has gained or lost in. Tungsten Oxidation Number.
From www.sciencephoto.com
Tungsten, atomic structure Stock Image C018/3755 Science Photo Tungsten Oxidation Number Tungsten is a chemical element with atomic number 74 which means there are 74 protons and 74 electrons in the atomic. It is defined as being the charge that. Tungsten exhibits a wide range of oxidation numbers from 0 to +6. The most common and stable state is +6, seen in compounds like tungsten trioxide (wo 3). Higher oxidation numbers. Tungsten Oxidation Number.
From achs-prod.acs.org
Atomic Layer Deposition of Ultrathin Tungsten Oxide Films from WH2(Cp)2 Tungsten Oxidation Number It is defined as being the charge that. A positive or negative integer that represents the number of electrons that an atom has gained or lost in a chemical compound. The most common and stable state is +6, seen in compounds like tungsten trioxide (wo 3). Tungsten and its alloys are widely used for filaments in older style (not energy. Tungsten Oxidation Number.
From www.researchgate.net
Oxidation tests on pure tungsten. Download Scientific Diagram Tungsten Oxidation Number The oxidation state of an atom is a measure of the degree of oxidation of an atom. Tungsten is a chemical element with atomic number 74 which means there are 74 protons and 74 electrons in the atomic. Tungsten and its alloys are widely used for filaments in older style (not energy saving). When present in compounds, tungsten exists mostly. Tungsten Oxidation Number.
From www.texaspowerfulsmart.com
Oxidation and Reduction of Tungsten and Tungsten Oxides Iron Oxide Tungsten Oxidation Number The oxidation state of an atom is a measure of the degree of oxidation of an atom. Tungsten is a chemical element with atomic number 74 which means there are 74 protons and 74 electrons in the atomic. Tungsten exhibits a wide range of oxidation numbers from 0 to +6. A positive or negative integer that represents the number of. Tungsten Oxidation Number.
From material-properties.org
Tungsten Periodic Table and Atomic Properties Tungsten Oxidation Number The most common and stable state is +6, seen in compounds like tungsten trioxide (wo 3). It is defined as being the charge that. Tungsten exhibits a wide range of oxidation numbers from 0 to +6. When present in compounds, tungsten exists mostly in the oxidation state vi. A positive or negative integer that represents the number of electrons that. Tungsten Oxidation Number.
From sharedocnow.blogspot.com
What Is The Oxidation Number Of Elements In Group 1 sharedoc Tungsten Oxidation Number When present in compounds, tungsten exists mostly in the oxidation state vi. A positive or negative integer that represents the number of electrons that an atom has gained or lost in a chemical compound. Higher oxidation numbers are relevant to its terrestrial occurrence and its biological roles. Tungsten and its alloys are widely used for filaments in older style (not. Tungsten Oxidation Number.
From www.researchgate.net
Tungsten 4f7/2, carbon I s and oxygen 1 s binding energies (eV) of some Tungsten Oxidation Number The oxidation state of an atom is a measure of the degree of oxidation of an atom. It is defined as being the charge that. Higher oxidation numbers are relevant to its terrestrial occurrence and its biological roles. Tungsten and its alloys are widely used for filaments in older style (not energy saving). A positive or negative integer that represents. Tungsten Oxidation Number.
From www.semanticscholar.org
Thermodynamics and of Tungsten Oxidation and Tungsten Oxide Tungsten Oxidation Number The most common and stable state is +6, seen in compounds like tungsten trioxide (wo 3). When present in compounds, tungsten exists mostly in the oxidation state vi. A positive or negative integer that represents the number of electrons that an atom has gained or lost in a chemical compound. Tungsten and its alloys are widely used for filaments in. Tungsten Oxidation Number.
From www.semanticscholar.org
Figure 2.1 from Thermodynamics and of Tungsten Oxidation and Tungsten Oxidation Number A positive or negative integer that represents the number of electrons that an atom has gained or lost in a chemical compound. The most common and stable state is +6, seen in compounds like tungsten trioxide (wo 3). Higher oxidation numbers are relevant to its terrestrial occurrence and its biological roles. It is defined as being the charge that. Tungsten. Tungsten Oxidation Number.
From blog.thepipingmart.com
Tungsten Oxide Properties A Brief Overview Tungsten Oxidation Number It is defined as being the charge that. Tungsten is a chemical element with atomic number 74 which means there are 74 protons and 74 electrons in the atomic. Tungsten exhibits a wide range of oxidation numbers from 0 to +6. Tungsten and its alloys are widely used for filaments in older style (not energy saving). When present in compounds,. Tungsten Oxidation Number.
From journals.sagepub.com
Structural and tribological properties of tungsten oxide thin film on a Tungsten Oxidation Number Tungsten and its alloys are widely used for filaments in older style (not energy saving). It is defined as being the charge that. Higher oxidation numbers are relevant to its terrestrial occurrence and its biological roles. Tungsten is a chemical element with atomic number 74 which means there are 74 protons and 74 electrons in the atomic. The most common. Tungsten Oxidation Number.
From www.toppr.com
The oxidation state of tungsten in Na2W4O13.10H2O is Tungsten Oxidation Number A positive or negative integer that represents the number of electrons that an atom has gained or lost in a chemical compound. It is defined as being the charge that. The most common and stable state is +6, seen in compounds like tungsten trioxide (wo 3). When present in compounds, tungsten exists mostly in the oxidation state vi. Tungsten and. Tungsten Oxidation Number.
From www.researchgate.net
(a) XRR on tungsten oxide layers grown for 30 min at different Tungsten Oxidation Number The oxidation state of an atom is a measure of the degree of oxidation of an atom. It is defined as being the charge that. Higher oxidation numbers are relevant to its terrestrial occurrence and its biological roles. When present in compounds, tungsten exists mostly in the oxidation state vi. Tungsten exhibits a wide range of oxidation numbers from 0. Tungsten Oxidation Number.
From www.chemistry-teaching-resources.com
chemistry picture Tungsten Oxidation Number It is defined as being the charge that. A positive or negative integer that represents the number of electrons that an atom has gained or lost in a chemical compound. When present in compounds, tungsten exists mostly in the oxidation state vi. The oxidation state of an atom is a measure of the degree of oxidation of an atom. Tungsten. Tungsten Oxidation Number.
From www.expii.com
Color and Oxidation State — Overview & Examples Expii Tungsten Oxidation Number The most common and stable state is +6, seen in compounds like tungsten trioxide (wo 3). When present in compounds, tungsten exists mostly in the oxidation state vi. It is defined as being the charge that. Tungsten and its alloys are widely used for filaments in older style (not energy saving). Tungsten exhibits a wide range of oxidation numbers from. Tungsten Oxidation Number.
From www.researchgate.net
Oxygentungsten phase diagram (redrawn from Ref. [36] and extended down Tungsten Oxidation Number Tungsten exhibits a wide range of oxidation numbers from 0 to +6. The most common and stable state is +6, seen in compounds like tungsten trioxide (wo 3). The oxidation state of an atom is a measure of the degree of oxidation of an atom. When present in compounds, tungsten exists mostly in the oxidation state vi. Tungsten and its. Tungsten Oxidation Number.
From www.youtube.com
How to find the Oxidation Number for W in the WO4 2 ion. (Tungstate Tungsten Oxidation Number Tungsten is a chemical element with atomic number 74 which means there are 74 protons and 74 electrons in the atomic. The oxidation state of an atom is a measure of the degree of oxidation of an atom. A positive or negative integer that represents the number of electrons that an atom has gained or lost in a chemical compound.. Tungsten Oxidation Number.
From www.mdpi.com
Catalysts Free FullText Boosting the Activity and Stability of Tungsten Oxidation Number The most common and stable state is +6, seen in compounds like tungsten trioxide (wo 3). It is defined as being the charge that. Higher oxidation numbers are relevant to its terrestrial occurrence and its biological roles. When present in compounds, tungsten exists mostly in the oxidation state vi. Tungsten exhibits a wide range of oxidation numbers from 0 to. Tungsten Oxidation Number.
From www.degruyter.com
Evolution of reduction process from tungsten oxide to ultrafine Tungsten Oxidation Number Tungsten exhibits a wide range of oxidation numbers from 0 to +6. When present in compounds, tungsten exists mostly in the oxidation state vi. Tungsten and its alloys are widely used for filaments in older style (not energy saving). It is defined as being the charge that. A positive or negative integer that represents the number of electrons that an. Tungsten Oxidation Number.
From www.mdpi.com
Coatings Free FullText MorphologyDependent NearInfrared Tungsten Oxidation Number When present in compounds, tungsten exists mostly in the oxidation state vi. A positive or negative integer that represents the number of electrons that an atom has gained or lost in a chemical compound. Tungsten exhibits a wide range of oxidation numbers from 0 to +6. The oxidation state of an atom is a measure of the degree of oxidation. Tungsten Oxidation Number.