Boiling Point Definition Quizlet . Therefore, the boiling point of a liquid depends on atmospheric pressure. The boiling point is the temperature at which a liquid's vapor pressure equals the external pressure surrounding it, causing the liquid to. Study with quizlet and memorize flashcards. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. Learn vocabulary, terms, and more with flashcards, games, and other study tools. The reason boiling point changes with elevation is because. The temperature at which the substance changes state from solid to liquid at atmospheric pressure. Study with quizlet and memorize flashcards containing terms like boiling point, boiling point, ionic compound and more. The boiling point becomes lower as the external pressure is reduced. The boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure.
from www.bartleby.com
The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. Study with quizlet and memorize flashcards containing terms like boiling point, boiling point, ionic compound and more. The boiling point becomes lower as the external pressure is reduced. The boiling point is the temperature at which a liquid's vapor pressure equals the external pressure surrounding it, causing the liquid to. Therefore, the boiling point of a liquid depends on atmospheric pressure. The temperature at which the substance changes state from solid to liquid at atmospheric pressure. The reason boiling point changes with elevation is because. Study with quizlet and memorize flashcards. Learn vocabulary, terms, and more with flashcards, games, and other study tools. The boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure.
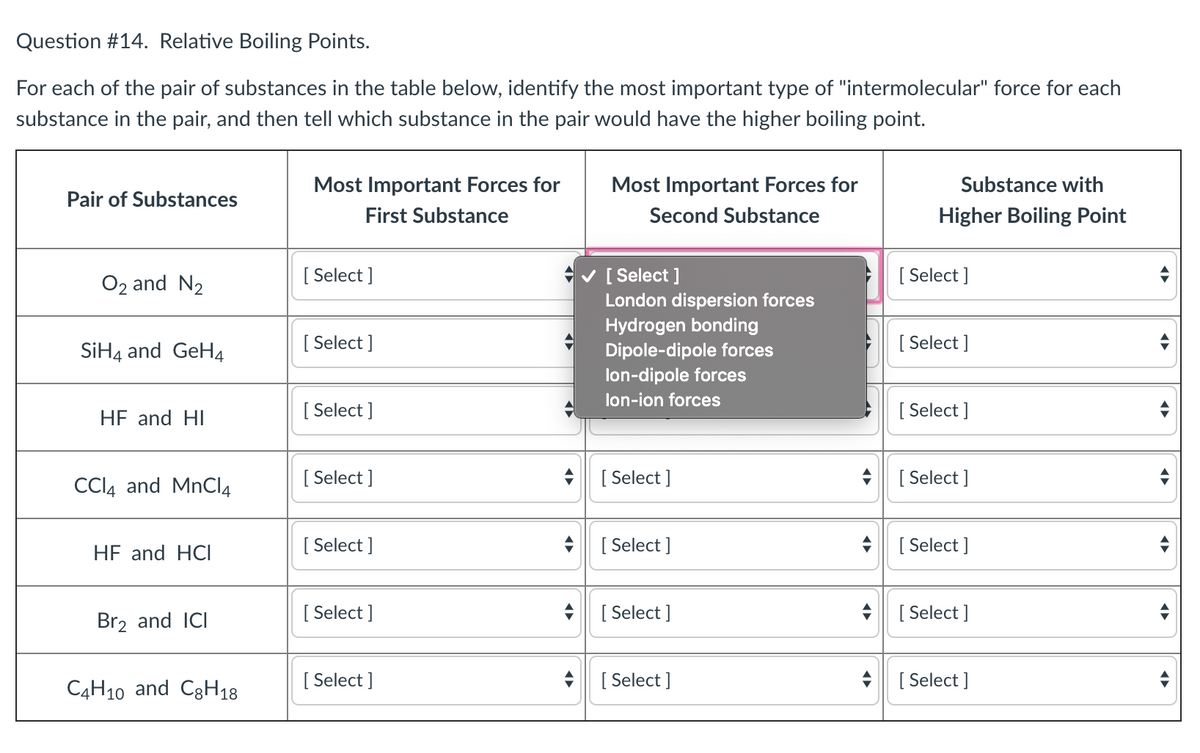
Answered Question 14. Relative Boiling Points.… bartleby
Boiling Point Definition Quizlet Study with quizlet and memorize flashcards. The boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. The boiling point is the temperature at which a liquid's vapor pressure equals the external pressure surrounding it, causing the liquid to. Learn vocabulary, terms, and more with flashcards, games, and other study tools. The temperature at which the substance changes state from solid to liquid at atmospheric pressure. The reason boiling point changes with elevation is because. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. The boiling point becomes lower as the external pressure is reduced. Study with quizlet and memorize flashcards containing terms like boiling point, boiling point, ionic compound and more. Therefore, the boiling point of a liquid depends on atmospheric pressure. Study with quizlet and memorize flashcards.
From chemistnotes.com
Boiling Point Definition, Boiling point as physical properties Boiling Point Definition Quizlet The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. The temperature at which the substance changes state from solid to liquid at atmospheric pressure. Study with quizlet and memorize flashcards containing terms like boiling point, boiling point, ionic compound and more. The boiling point becomes lower as the. Boiling Point Definition Quizlet.
From userenginelegumes.z14.web.core.windows.net
The Normal Boiling Point Boiling Point Definition Quizlet Study with quizlet and memorize flashcards. The temperature at which the substance changes state from solid to liquid at atmospheric pressure. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. The reason boiling point changes with elevation is because. Study with quizlet and memorize flashcards containing terms like. Boiling Point Definition Quizlet.
From www.chemicalslearning.com
Boiling Point ElevationDefinition, Formula with Examples Boiling Point Definition Quizlet Learn vocabulary, terms, and more with flashcards, games, and other study tools. The boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. Study with quizlet and memorize flashcards. The temperature at which the substance changes state from solid to liquid at atmospheric pressure. Therefore, the boiling point of a liquid depends on atmospheric pressure.. Boiling Point Definition Quizlet.
From www.thoughtco.com
Normal Boiling Point Definition (Chemistry) Boiling Point Definition Quizlet The boiling point is the temperature at which a liquid's vapor pressure equals the external pressure surrounding it, causing the liquid to. Study with quizlet and memorize flashcards. The temperature at which the substance changes state from solid to liquid at atmospheric pressure. Therefore, the boiling point of a liquid depends on atmospheric pressure. The boiling point is the temperature. Boiling Point Definition Quizlet.
From safrole.com
Boiling point definition Safrole Boiling Point Definition Quizlet The boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. The reason boiling point changes with elevation is because. The boiling point is the temperature at which a liquid's vapor pressure equals the. Boiling Point Definition Quizlet.
From www.youtube.com
Boiling point — BOILING POINT definition YouTube Boiling Point Definition Quizlet The boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. Therefore, the boiling point of a liquid depends on atmospheric pressure. Study with quizlet and memorize flashcards. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. Study with quizlet and memorize flashcards. Boiling Point Definition Quizlet.
From chemistnotes.com
Elevation of Boiling Point Definition/Equation/Molal Boiling Point Boiling Point Definition Quizlet The boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. The temperature at which the substance changes state from solid to liquid at atmospheric pressure. Study with quizlet and memorize flashcards containing terms. Boiling Point Definition Quizlet.
From www.slideserve.com
PPT Molecular Theory and the the Nature of Fluids PowerPoint Boiling Point Definition Quizlet The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. Therefore, the boiling point of a liquid depends on atmospheric pressure. The reason boiling point changes with elevation is because. Study with quizlet and memorize flashcards. The boiling point of water is the temperature where the liquid’s vapor pressure. Boiling Point Definition Quizlet.
From www.slideserve.com
PPT boiling point PowerPoint Presentation, free download ID2402961 Boiling Point Definition Quizlet Learn vocabulary, terms, and more with flashcards, games, and other study tools. The boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. The temperature at which the substance changes state from solid to liquid at atmospheric pressure. Study with quizlet and memorize flashcards containing terms like boiling point, boiling point, ionic compound and more.. Boiling Point Definition Quizlet.
From www.healthbenefitstimes.com
Boiling Point Definition of Boiling Point Boiling Point Definition Quizlet Learn vocabulary, terms, and more with flashcards, games, and other study tools. The boiling point becomes lower as the external pressure is reduced. The temperature at which the substance changes state from solid to liquid at atmospheric pressure. The reason boiling point changes with elevation is because. Therefore, the boiling point of a liquid depends on atmospheric pressure. Study with. Boiling Point Definition Quizlet.
From www.vedantu.com
Boiling Point Elevation Learn Important Terms and Concepts Boiling Point Definition Quizlet Study with quizlet and memorize flashcards. The boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. The temperature at which the substance changes state from solid to liquid at atmospheric pressure. The boiling. Boiling Point Definition Quizlet.
From www.masterorganicchemistry.com
Boiling Point Quizzes Master Organic Chemistry Boiling Point Definition Quizlet The reason boiling point changes with elevation is because. The temperature at which the substance changes state from solid to liquid at atmospheric pressure. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Study with quizlet and memorize flashcards. The boiling point is the temperature at which a liquid's vapor pressure equals the external pressure surrounding it,. Boiling Point Definition Quizlet.
From progressivesmartquiz.blogspot.com
Boiling Point In Celcius Progressive Smart Quiz Boiling Point Definition Quizlet Therefore, the boiling point of a liquid depends on atmospheric pressure. The temperature at which the substance changes state from solid to liquid at atmospheric pressure. Study with quizlet and memorize flashcards. Learn vocabulary, terms, and more with flashcards, games, and other study tools. The reason boiling point changes with elevation is because. The boiling point is the temperature at. Boiling Point Definition Quizlet.
From byjus.com
What is the difference between melting point and boiling point Boiling Point Definition Quizlet The temperature at which the substance changes state from solid to liquid at atmospheric pressure. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. Therefore, the boiling point of a liquid depends on atmospheric pressure. Study with quizlet and memorize flashcards containing terms like boiling point, boiling point,. Boiling Point Definition Quizlet.
From www.bartleby.com
Answered Question 14. Relative Boiling Points.… bartleby Boiling Point Definition Quizlet Study with quizlet and memorize flashcards. The boiling point is the temperature at which a liquid's vapor pressure equals the external pressure surrounding it, causing the liquid to. The reason boiling point changes with elevation is because. Study with quizlet and memorize flashcards containing terms like boiling point, boiling point, ionic compound and more. Therefore, the boiling point of a. Boiling Point Definition Quizlet.
From pediaa.com
Difference Between Vapor Pressure and Boiling Point Definition Boiling Point Definition Quizlet The boiling point becomes lower as the external pressure is reduced. The reason boiling point changes with elevation is because. The boiling point is the temperature at which a liquid's vapor pressure equals the external pressure surrounding it, causing the liquid to. Learn vocabulary, terms, and more with flashcards, games, and other study tools. The temperature at which the substance. Boiling Point Definition Quizlet.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples Boiling Point Definition Quizlet Study with quizlet and memorize flashcards. The reason boiling point changes with elevation is because. The boiling point is the temperature at which a liquid's vapor pressure equals the external pressure surrounding it, causing the liquid to. Study with quizlet and memorize flashcards containing terms like boiling point, boiling point, ionic compound and more. The boiling point of water is. Boiling Point Definition Quizlet.
From elecschem.com
The Importance of Understanding Boiling Point Diagrams for Chemical Boiling Point Definition Quizlet The temperature at which the substance changes state from solid to liquid at atmospheric pressure. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. The boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. Study with quizlet and memorize flashcards containing terms. Boiling Point Definition Quizlet.
From gamesmartz.com
Boiling Point Definition & Image GameSmartz Boiling Point Definition Quizlet Therefore, the boiling point of a liquid depends on atmospheric pressure. Study with quizlet and memorize flashcards. The temperature at which the substance changes state from solid to liquid at atmospheric pressure. Study with quizlet and memorize flashcards containing terms like boiling point, boiling point, ionic compound and more. The reason boiling point changes with elevation is because. The boiling. Boiling Point Definition Quizlet.
From www.youtube.com
Difference between Melting point / Boiling point & Freezing point Boiling Point Definition Quizlet The temperature at which the substance changes state from solid to liquid at atmospheric pressure. The reason boiling point changes with elevation is because. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Study with quizlet and memorize flashcards. The boiling point becomes lower as the external pressure is reduced. The boiling point is the temperature at. Boiling Point Definition Quizlet.
From www.youtube.com
Definition of Boiling Point / Melting Point / Freezing Point / Flash Boiling Point Definition Quizlet The boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. Study with quizlet and memorize flashcards containing terms like boiling point, boiling point, ionic compound and more. The reason boiling point changes with elevation is because. Study with quizlet and memorize flashcards. The boiling point is the temperature at which a liquid's vapor pressure. Boiling Point Definition Quizlet.
From matmake.com
Boiling Point Definition, Measurements, and Applications Boiling Point Definition Quizlet The boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. Study with quizlet and memorize flashcards containing terms like boiling point, boiling point, ionic compound and more. The reason boiling point changes with elevation is because. The boiling point is the temperature at which a liquid's vapor pressure equals the external pressure surrounding it,. Boiling Point Definition Quizlet.
From quizlet.com
Freezing and Boiling Point Graph Diagram Quizlet Boiling Point Definition Quizlet Study with quizlet and memorize flashcards. Study with quizlet and memorize flashcards containing terms like boiling point, boiling point, ionic compound and more. The reason boiling point changes with elevation is because. The boiling point is the temperature at which a liquid's vapor pressure equals the external pressure surrounding it, causing the liquid to. Therefore, the boiling point of a. Boiling Point Definition Quizlet.
From gamesmartz.com
Boiling Point Elevation Definition & Image GameSmartz Boiling Point Definition Quizlet Study with quizlet and memorize flashcards. The boiling point becomes lower as the external pressure is reduced. Learn vocabulary, terms, and more with flashcards, games, and other study tools. The boiling point is the temperature at which a liquid's vapor pressure equals the external pressure surrounding it, causing the liquid to. Study with quizlet and memorize flashcards containing terms like. Boiling Point Definition Quizlet.
From diagramfricanofc.z21.web.core.windows.net
Initial Boiling Point And Final Boiling Point Boiling Point Definition Quizlet Therefore, the boiling point of a liquid depends on atmospheric pressure. The boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. The temperature at which the substance changes state from solid to liquid at atmospheric pressure. The boiling point is the temperature at which a liquid's vapor pressure equals the external pressure surrounding it,. Boiling Point Definition Quizlet.
From studylibplimsoll.z21.web.core.windows.net
Blood Boils At What Temperature Boiling Point Definition Quizlet The boiling point becomes lower as the external pressure is reduced. Therefore, the boiling point of a liquid depends on atmospheric pressure. The reason boiling point changes with elevation is because. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. Study with quizlet and memorize flashcards containing terms. Boiling Point Definition Quizlet.
From www.worksheetsplanet.com
What is Boiling Point Boiling Point Definition Quizlet The reason boiling point changes with elevation is because. The boiling point is the temperature at which a liquid's vapor pressure equals the external pressure surrounding it, causing the liquid to. Therefore, the boiling point of a liquid depends on atmospheric pressure. Study with quizlet and memorize flashcards containing terms like boiling point, boiling point, ionic compound and more. The. Boiling Point Definition Quizlet.
From www.thoughtco.com
Definition of Boiling Point in Chemistry Boiling Point Definition Quizlet Study with quizlet and memorize flashcards. The boiling point is the temperature at which a liquid's vapor pressure equals the external pressure surrounding it, causing the liquid to. The boiling point of water is the temperature where the liquid’s vapor pressure equals atmospheric pressure. Therefore, the boiling point of a liquid depends on atmospheric pressure. The boiling point is the. Boiling Point Definition Quizlet.
From vdocuments.mx
States, Boiling Point, Melting Point, and Solubility · Boiling Point Boiling Point Definition Quizlet Study with quizlet and memorize flashcards. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. The boiling point is the temperature at which a liquid's vapor pressure equals the external pressure surrounding it, causing the liquid to. The boiling point becomes lower as the external pressure is reduced.. Boiling Point Definition Quizlet.
From www.slideserve.com
PPT Boiling Point Notes PowerPoint Presentation, free download ID Boiling Point Definition Quizlet Learn vocabulary, terms, and more with flashcards, games, and other study tools. The reason boiling point changes with elevation is because. The temperature at which the substance changes state from solid to liquid at atmospheric pressure. The boiling point is the temperature at which a liquid's vapor pressure equals the external pressure surrounding it, causing the liquid to. Therefore, the. Boiling Point Definition Quizlet.
From www.slideserve.com
PPT Freezing/Melting and Boiling Points PowerPoint Presentation, free Boiling Point Definition Quizlet The temperature at which the substance changes state from solid to liquid at atmospheric pressure. The boiling point is the temperature at which a liquid's vapor pressure equals the external pressure surrounding it, causing the liquid to. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. Learn vocabulary,. Boiling Point Definition Quizlet.
From www.youtube.com
What is boiling point what is the boiling point Factors affecting Boiling Point Definition Quizlet Learn vocabulary, terms, and more with flashcards, games, and other study tools. Therefore, the boiling point of a liquid depends on atmospheric pressure. The temperature at which the substance changes state from solid to liquid at atmospheric pressure. Study with quizlet and memorize flashcards containing terms like boiling point, boiling point, ionic compound and more. The boiling point is the. Boiling Point Definition Quizlet.
From www.slideserve.com
PPT Physical and Chemical Properties PowerPoint Presentation, free Boiling Point Definition Quizlet The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. The boiling point is the temperature at which a liquid's vapor pressure equals the external pressure surrounding it, causing the liquid to. Therefore, the boiling point of a liquid depends on atmospheric pressure. Study with quizlet and memorize flashcards.. Boiling Point Definition Quizlet.
From www.animalia-life.club
Boiling Point Of Water For Kids Boiling Point Definition Quizlet The reason boiling point changes with elevation is because. Learn vocabulary, terms, and more with flashcards, games, and other study tools. The boiling point is the temperature at which a liquid's vapor pressure equals the external pressure surrounding it, causing the liquid to. The boiling point is the temperature at which the vapor pressure of a liquid equals the external. Boiling Point Definition Quizlet.
From www.youtube.com
What is Boiling Point Concept of Boiling Point Boiling Point Boiling Point Definition Quizlet The reason boiling point changes with elevation is because. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. The boiling point is the temperature at which a liquid's vapor pressure equals the external pressure surrounding it, causing the liquid to. Therefore, the boiling point of a liquid depends. Boiling Point Definition Quizlet.