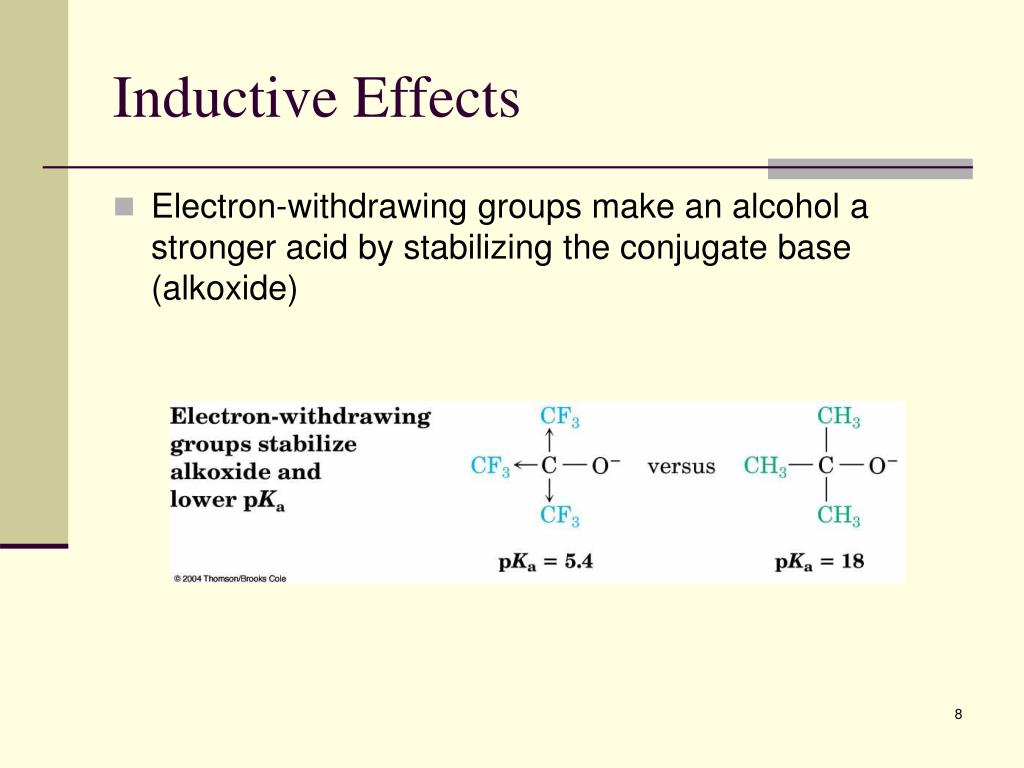
Inductive Effect Electron Withdrawing Groups . Electron withdrawing groups have an atom with a slight positive or full positive charge directly attached to a benzene ring. If the atom or the group donates electron density, it is said to have a positive inductive effect (+i). Atoms or groups that pull electron density from another atom or a group through sigma bond (s),. There are two categories of inductive effects:
from www.slideserve.com
Atoms or groups that pull electron density from another atom or a group through sigma bond (s),. If the atom or the group donates electron density, it is said to have a positive inductive effect (+i). Electron withdrawing groups have an atom with a slight positive or full positive charge directly attached to a benzene ring. There are two categories of inductive effects:
PPT Alcohols and Phenols PowerPoint Presentation, free download ID
Inductive Effect Electron Withdrawing Groups Atoms or groups that pull electron density from another atom or a group through sigma bond (s),. There are two categories of inductive effects: Atoms or groups that pull electron density from another atom or a group through sigma bond (s),. Electron withdrawing groups have an atom with a slight positive or full positive charge directly attached to a benzene ring. If the atom or the group donates electron density, it is said to have a positive inductive effect (+i).
From www.numerade.com
SOLVED Identify the groups circled as electron withdrawing or electron Inductive Effect Electron Withdrawing Groups If the atom or the group donates electron density, it is said to have a positive inductive effect (+i). Electron withdrawing groups have an atom with a slight positive or full positive charge directly attached to a benzene ring. Atoms or groups that pull electron density from another atom or a group through sigma bond (s),. There are two categories. Inductive Effect Electron Withdrawing Groups.
From lambdageeks.com
Are Aldehydes Electron Withdrawing9 Facts You Should Know Lambda Geeks Inductive Effect Electron Withdrawing Groups There are two categories of inductive effects: Electron withdrawing groups have an atom with a slight positive or full positive charge directly attached to a benzene ring. Atoms or groups that pull electron density from another atom or a group through sigma bond (s),. If the atom or the group donates electron density, it is said to have a positive. Inductive Effect Electron Withdrawing Groups.
From www.chegg.com
Solved Inductive effects can be either electron donating Inductive Effect Electron Withdrawing Groups There are two categories of inductive effects: If the atom or the group donates electron density, it is said to have a positive inductive effect (+i). Atoms or groups that pull electron density from another atom or a group through sigma bond (s),. Electron withdrawing groups have an atom with a slight positive or full positive charge directly attached to. Inductive Effect Electron Withdrawing Groups.
From www.chemistrysteps.com
Basicity of Amines Chemistry Steps Inductive Effect Electron Withdrawing Groups Electron withdrawing groups have an atom with a slight positive or full positive charge directly attached to a benzene ring. There are two categories of inductive effects: Atoms or groups that pull electron density from another atom or a group through sigma bond (s),. If the atom or the group donates electron density, it is said to have a positive. Inductive Effect Electron Withdrawing Groups.
From www.pinterest.com
صورة ذات صلة Organic chemistry, Organic chem, Chemistry Inductive Effect Electron Withdrawing Groups There are two categories of inductive effects: Electron withdrawing groups have an atom with a slight positive or full positive charge directly attached to a benzene ring. If the atom or the group donates electron density, it is said to have a positive inductive effect (+i). Atoms or groups that pull electron density from another atom or a group through. Inductive Effect Electron Withdrawing Groups.
From slideplayer.com
CARBOXYLIC ACID AND ITS DERIVATIVES ppt download Inductive Effect Electron Withdrawing Groups Atoms or groups that pull electron density from another atom or a group through sigma bond (s),. Electron withdrawing groups have an atom with a slight positive or full positive charge directly attached to a benzene ring. If the atom or the group donates electron density, it is said to have a positive inductive effect (+i). There are two categories. Inductive Effect Electron Withdrawing Groups.
From byjus.com
Inductive Effect Types of Inductive Effect, Applications, Stability Inductive Effect Electron Withdrawing Groups Electron withdrawing groups have an atom with a slight positive or full positive charge directly attached to a benzene ring. There are two categories of inductive effects: Atoms or groups that pull electron density from another atom or a group through sigma bond (s),. If the atom or the group donates electron density, it is said to have a positive. Inductive Effect Electron Withdrawing Groups.
From slideplayer.com
NEW CHAPTER BASIC CONCEPTS BINDING AND RESONANCE. ppt download Inductive Effect Electron Withdrawing Groups Atoms or groups that pull electron density from another atom or a group through sigma bond (s),. There are two categories of inductive effects: If the atom or the group donates electron density, it is said to have a positive inductive effect (+i). Electron withdrawing groups have an atom with a slight positive or full positive charge directly attached to. Inductive Effect Electron Withdrawing Groups.
From www.youtube.com
Organic ChemistryInductive effectElectron Withdrawing Groups YouTube Inductive Effect Electron Withdrawing Groups If the atom or the group donates electron density, it is said to have a positive inductive effect (+i). Electron withdrawing groups have an atom with a slight positive or full positive charge directly attached to a benzene ring. Atoms or groups that pull electron density from another atom or a group through sigma bond (s),. There are two categories. Inductive Effect Electron Withdrawing Groups.
From www.chemistrysteps.com
Basicity of Amines Chemistry Steps Inductive Effect Electron Withdrawing Groups There are two categories of inductive effects: If the atom or the group donates electron density, it is said to have a positive inductive effect (+i). Electron withdrawing groups have an atom with a slight positive or full positive charge directly attached to a benzene ring. Atoms or groups that pull electron density from another atom or a group through. Inductive Effect Electron Withdrawing Groups.
From www.slideserve.com
PPT Chapter 16 Chemistry of Benzene Electrophilic Aromatic Inductive Effect Electron Withdrawing Groups There are two categories of inductive effects: Electron withdrawing groups have an atom with a slight positive or full positive charge directly attached to a benzene ring. Atoms or groups that pull electron density from another atom or a group through sigma bond (s),. If the atom or the group donates electron density, it is said to have a positive. Inductive Effect Electron Withdrawing Groups.
From www.youtube.com
27.02 Resonance and Inductive Effects YouTube Inductive Effect Electron Withdrawing Groups There are two categories of inductive effects: Electron withdrawing groups have an atom with a slight positive or full positive charge directly attached to a benzene ring. If the atom or the group donates electron density, it is said to have a positive inductive effect (+i). Atoms or groups that pull electron density from another atom or a group through. Inductive Effect Electron Withdrawing Groups.
From chemistry.stackexchange.com
organic chemistry Why are halogens ortho para directing even though Inductive Effect Electron Withdrawing Groups Electron withdrawing groups have an atom with a slight positive or full positive charge directly attached to a benzene ring. If the atom or the group donates electron density, it is said to have a positive inductive effect (+i). Atoms or groups that pull electron density from another atom or a group through sigma bond (s),. There are two categories. Inductive Effect Electron Withdrawing Groups.
From chemistnotes.com
Inductive Effect Definitions/ Examples Chemistry Notes Inductive Effect Electron Withdrawing Groups If the atom or the group donates electron density, it is said to have a positive inductive effect (+i). Atoms or groups that pull electron density from another atom or a group through sigma bond (s),. Electron withdrawing groups have an atom with a slight positive or full positive charge directly attached to a benzene ring. There are two categories. Inductive Effect Electron Withdrawing Groups.
From www.slideserve.com
PPT Alcohols and Phenols PowerPoint Presentation, free download ID Inductive Effect Electron Withdrawing Groups If the atom or the group donates electron density, it is said to have a positive inductive effect (+i). Electron withdrawing groups have an atom with a slight positive or full positive charge directly attached to a benzene ring. There are two categories of inductive effects: Atoms or groups that pull electron density from another atom or a group through. Inductive Effect Electron Withdrawing Groups.
From slideplayer.com
Fundamentals of Organic Chemistry CHAPTER 1. INTRODUCTION ppt download Inductive Effect Electron Withdrawing Groups There are two categories of inductive effects: Atoms or groups that pull electron density from another atom or a group through sigma bond (s),. Electron withdrawing groups have an atom with a slight positive or full positive charge directly attached to a benzene ring. If the atom or the group donates electron density, it is said to have a positive. Inductive Effect Electron Withdrawing Groups.
From slideplayer.com
NEW CHAPTER BASIC CONCEPTS BINDING AND RESONANCE. ppt download Inductive Effect Electron Withdrawing Groups Atoms or groups that pull electron density from another atom or a group through sigma bond (s),. If the atom or the group donates electron density, it is said to have a positive inductive effect (+i). There are two categories of inductive effects: Electron withdrawing groups have an atom with a slight positive or full positive charge directly attached to. Inductive Effect Electron Withdrawing Groups.
From www.clutchprep.com
Electron Withdrawing Groups Organic Chemistry Video Clutch Prep Inductive Effect Electron Withdrawing Groups Electron withdrawing groups have an atom with a slight positive or full positive charge directly attached to a benzene ring. There are two categories of inductive effects: If the atom or the group donates electron density, it is said to have a positive inductive effect (+i). Atoms or groups that pull electron density from another atom or a group through. Inductive Effect Electron Withdrawing Groups.
From quizlet.com
Electron Withdrawing Groups Diagram Quizlet Inductive Effect Electron Withdrawing Groups If the atom or the group donates electron density, it is said to have a positive inductive effect (+i). Atoms or groups that pull electron density from another atom or a group through sigma bond (s),. There are two categories of inductive effects: Electron withdrawing groups have an atom with a slight positive or full positive charge directly attached to. Inductive Effect Electron Withdrawing Groups.
From slideplayer.com
Introduction to organic chemistry Part I ppt download Inductive Effect Electron Withdrawing Groups If the atom or the group donates electron density, it is said to have a positive inductive effect (+i). There are two categories of inductive effects: Electron withdrawing groups have an atom with a slight positive or full positive charge directly attached to a benzene ring. Atoms or groups that pull electron density from another atom or a group through. Inductive Effect Electron Withdrawing Groups.
From www.slideserve.com
PPT Electrophilic Aromatic Substitution PowerPoint Presentation, free Inductive Effect Electron Withdrawing Groups Atoms or groups that pull electron density from another atom or a group through sigma bond (s),. There are two categories of inductive effects: If the atom or the group donates electron density, it is said to have a positive inductive effect (+i). Electron withdrawing groups have an atom with a slight positive or full positive charge directly attached to. Inductive Effect Electron Withdrawing Groups.
From www.numerade.com
SOLVED Identify the groups circled as electron withdrawing or electron Inductive Effect Electron Withdrawing Groups There are two categories of inductive effects: Electron withdrawing groups have an atom with a slight positive or full positive charge directly attached to a benzene ring. If the atom or the group donates electron density, it is said to have a positive inductive effect (+i). Atoms or groups that pull electron density from another atom or a group through. Inductive Effect Electron Withdrawing Groups.
From chem.libretexts.org
Inductive Effects of Alkyl Groups Chemistry LibreTexts Inductive Effect Electron Withdrawing Groups Atoms or groups that pull electron density from another atom or a group through sigma bond (s),. Electron withdrawing groups have an atom with a slight positive or full positive charge directly attached to a benzene ring. There are two categories of inductive effects: If the atom or the group donates electron density, it is said to have a positive. Inductive Effect Electron Withdrawing Groups.
From slideplayer.com
Introduction to organic chemistry Part I ppt download Inductive Effect Electron Withdrawing Groups Atoms or groups that pull electron density from another atom or a group through sigma bond (s),. There are two categories of inductive effects: If the atom or the group donates electron density, it is said to have a positive inductive effect (+i). Electron withdrawing groups have an atom with a slight positive or full positive charge directly attached to. Inductive Effect Electron Withdrawing Groups.
From www.youtube.com
What is Electron Withdrawing Groups ? Inductive effect & Acidity Inductive Effect Electron Withdrawing Groups Electron withdrawing groups have an atom with a slight positive or full positive charge directly attached to a benzene ring. There are two categories of inductive effects: If the atom or the group donates electron density, it is said to have a positive inductive effect (+i). Atoms or groups that pull electron density from another atom or a group through. Inductive Effect Electron Withdrawing Groups.
From www.toppr.com
A ORGANIC CHEMISTRY GENERAL ORGANIC TYPES OF INDUCTIVE EFFECT divided Inductive Effect Electron Withdrawing Groups There are two categories of inductive effects: Electron withdrawing groups have an atom with a slight positive or full positive charge directly attached to a benzene ring. If the atom or the group donates electron density, it is said to have a positive inductive effect (+i). Atoms or groups that pull electron density from another atom or a group through. Inductive Effect Electron Withdrawing Groups.
From www.youtube.com
Inductive effect order l and +l order trick YouTube Inductive Effect Electron Withdrawing Groups If the atom or the group donates electron density, it is said to have a positive inductive effect (+i). Atoms or groups that pull electron density from another atom or a group through sigma bond (s),. Electron withdrawing groups have an atom with a slight positive or full positive charge directly attached to a benzene ring. There are two categories. Inductive Effect Electron Withdrawing Groups.
From www.numerade.com
SOLVED Identify the groups circled as electron withdrawing or electron Inductive Effect Electron Withdrawing Groups If the atom or the group donates electron density, it is said to have a positive inductive effect (+i). There are two categories of inductive effects: Atoms or groups that pull electron density from another atom or a group through sigma bond (s),. Electron withdrawing groups have an atom with a slight positive or full positive charge directly attached to. Inductive Effect Electron Withdrawing Groups.
From www.youtube.com
Organic chemistry Inductive Effect +I & I effect electron Inductive Effect Electron Withdrawing Groups Electron withdrawing groups have an atom with a slight positive or full positive charge directly attached to a benzene ring. If the atom or the group donates electron density, it is said to have a positive inductive effect (+i). There are two categories of inductive effects: Atoms or groups that pull electron density from another atom or a group through. Inductive Effect Electron Withdrawing Groups.
From electronic--racket.blogspot.com
How To Identify Electron Withdrawing And Donating Groups Inductive Effect Electron Withdrawing Groups Atoms or groups that pull electron density from another atom or a group through sigma bond (s),. Electron withdrawing groups have an atom with a slight positive or full positive charge directly attached to a benzene ring. If the atom or the group donates electron density, it is said to have a positive inductive effect (+i). There are two categories. Inductive Effect Electron Withdrawing Groups.
From slideplayer.com
NEW CHAPTER BASIC CONCEPTS BINDING AND RESONANCE. ppt download Inductive Effect Electron Withdrawing Groups Atoms or groups that pull electron density from another atom or a group through sigma bond (s),. Electron withdrawing groups have an atom with a slight positive or full positive charge directly attached to a benzene ring. There are two categories of inductive effects: If the atom or the group donates electron density, it is said to have a positive. Inductive Effect Electron Withdrawing Groups.
From www.chemistrystudent.com
Amines (ALevel) ChemistryStudent Inductive Effect Electron Withdrawing Groups There are two categories of inductive effects: Electron withdrawing groups have an atom with a slight positive or full positive charge directly attached to a benzene ring. If the atom or the group donates electron density, it is said to have a positive inductive effect (+i). Atoms or groups that pull electron density from another atom or a group through. Inductive Effect Electron Withdrawing Groups.
From www.slideserve.com
PPT Chapter 16 Chemistry of Benzene Electrophilic Aromatic Inductive Effect Electron Withdrawing Groups Atoms or groups that pull electron density from another atom or a group through sigma bond (s),. There are two categories of inductive effects: Electron withdrawing groups have an atom with a slight positive or full positive charge directly attached to a benzene ring. If the atom or the group donates electron density, it is said to have a positive. Inductive Effect Electron Withdrawing Groups.
From www.science.org
Electroinductive effect Electrodes as functional groups with tunable Inductive Effect Electron Withdrawing Groups If the atom or the group donates electron density, it is said to have a positive inductive effect (+i). There are two categories of inductive effects: Atoms or groups that pull electron density from another atom or a group through sigma bond (s),. Electron withdrawing groups have an atom with a slight positive or full positive charge directly attached to. Inductive Effect Electron Withdrawing Groups.
From slidetodoc.com
Introduction to AcidBase Chemistry BrnstedLowry Definition of Acids Inductive Effect Electron Withdrawing Groups There are two categories of inductive effects: Atoms or groups that pull electron density from another atom or a group through sigma bond (s),. Electron withdrawing groups have an atom with a slight positive or full positive charge directly attached to a benzene ring. If the atom or the group donates electron density, it is said to have a positive. Inductive Effect Electron Withdrawing Groups.