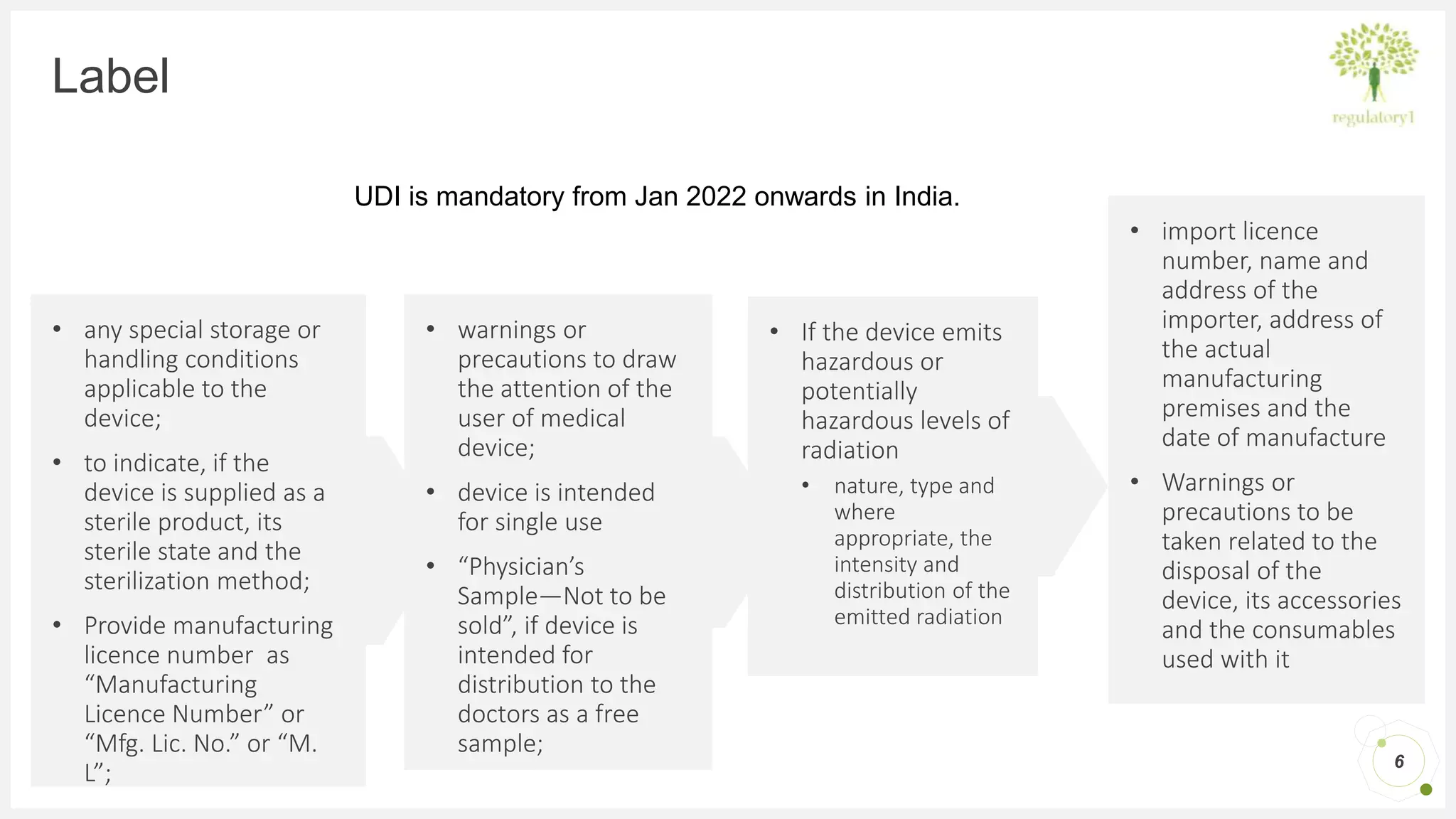
Medical Device Labeling Requirements India . Discover the essential medical device labeling requirements in india as per the legal metrology act, 2009, and the medical. 89 rows medical device & diagnostics. In india, all medical devices are regulated under the drugs & cosmetics act, 1940 and medical devices rules, 2017 made thereunder. Ministry of health & family welfare, government of india has released the medical device rules, 2017, effective from 1 st january, 2018 for. Medical device manufacturers or importers must provide the labels of the device and ifu, in compliance with chapter vi of labelling of medical devices rules (mdr) 2017. In addition to cdsco regulations, the legal metrology packaged commodity act of 2009 and rules of 2011 is a mandatory license for the manufacture of medical and in. Alternatively the label shall bear the shelf life of the product. Medical device labeling requirements in india are prescribed in chapter vi of the medical devices rules, 2017. He central drugs standard control organisation(cdsco)under directorate general of health services,ministry of health & family.
from www.slideshare.net
In india, all medical devices are regulated under the drugs & cosmetics act, 1940 and medical devices rules, 2017 made thereunder. Medical device manufacturers or importers must provide the labels of the device and ifu, in compliance with chapter vi of labelling of medical devices rules (mdr) 2017. Alternatively the label shall bear the shelf life of the product. Discover the essential medical device labeling requirements in india as per the legal metrology act, 2009, and the medical. He central drugs standard control organisation(cdsco)under directorate general of health services,ministry of health & family. 89 rows medical device & diagnostics. Medical device labeling requirements in india are prescribed in chapter vi of the medical devices rules, 2017. Ministry of health & family welfare, government of india has released the medical device rules, 2017, effective from 1 st january, 2018 for. In addition to cdsco regulations, the legal metrology packaged commodity act of 2009 and rules of 2011 is a mandatory license for the manufacture of medical and in.
Labelling requirements india PPT
Medical Device Labeling Requirements India In addition to cdsco regulations, the legal metrology packaged commodity act of 2009 and rules of 2011 is a mandatory license for the manufacture of medical and in. Medical device labeling requirements in india are prescribed in chapter vi of the medical devices rules, 2017. Discover the essential medical device labeling requirements in india as per the legal metrology act, 2009, and the medical. Medical device manufacturers or importers must provide the labels of the device and ifu, in compliance with chapter vi of labelling of medical devices rules (mdr) 2017. Ministry of health & family welfare, government of india has released the medical device rules, 2017, effective from 1 st january, 2018 for. He central drugs standard control organisation(cdsco)under directorate general of health services,ministry of health & family. In india, all medical devices are regulated under the drugs & cosmetics act, 1940 and medical devices rules, 2017 made thereunder. Alternatively the label shall bear the shelf life of the product. In addition to cdsco regulations, the legal metrology packaged commodity act of 2009 and rules of 2011 is a mandatory license for the manufacture of medical and in. 89 rows medical device & diagnostics.
From www.pinterest.jp
Épinglé sur Pharmaceutical Labeling Medical Device Labeling Requirements India In addition to cdsco regulations, the legal metrology packaged commodity act of 2009 and rules of 2011 is a mandatory license for the manufacture of medical and in. 89 rows medical device & diagnostics. He central drugs standard control organisation(cdsco)under directorate general of health services,ministry of health & family. Medical device labeling requirements in india are prescribed in chapter vi. Medical Device Labeling Requirements India.
From abr.com
Label Compliance AB&R® (American Barcode and RFID) Medical Device Labeling Requirements India Medical device labeling requirements in india are prescribed in chapter vi of the medical devices rules, 2017. In india, all medical devices are regulated under the drugs & cosmetics act, 1940 and medical devices rules, 2017 made thereunder. 89 rows medical device & diagnostics. Ministry of health & family welfare, government of india has released the medical device rules, 2017,. Medical Device Labeling Requirements India.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Labeling Requirements India Medical device manufacturers or importers must provide the labels of the device and ifu, in compliance with chapter vi of labelling of medical devices rules (mdr) 2017. In india, all medical devices are regulated under the drugs & cosmetics act, 1940 and medical devices rules, 2017 made thereunder. 89 rows medical device & diagnostics. Medical device labeling requirements in india. Medical Device Labeling Requirements India.
From old.sermitsiaq.ag
Medical Device Label Template Medical Device Labeling Requirements India Ministry of health & family welfare, government of india has released the medical device rules, 2017, effective from 1 st january, 2018 for. He central drugs standard control organisation(cdsco)under directorate general of health services,ministry of health & family. Discover the essential medical device labeling requirements in india as per the legal metrology act, 2009, and the medical. 89 rows medical. Medical Device Labeling Requirements India.
From labelservice.co.uk
Medical Device Labels, Medical Device Labelling Labelservice Medical Device Labeling Requirements India Discover the essential medical device labeling requirements in india as per the legal metrology act, 2009, and the medical. 89 rows medical device & diagnostics. Medical device manufacturers or importers must provide the labels of the device and ifu, in compliance with chapter vi of labelling of medical devices rules (mdr) 2017. Ministry of health & family welfare, government of. Medical Device Labeling Requirements India.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Medical Device Labeling Requirements India Ministry of health & family welfare, government of india has released the medical device rules, 2017, effective from 1 st january, 2018 for. Medical device manufacturers or importers must provide the labels of the device and ifu, in compliance with chapter vi of labelling of medical devices rules (mdr) 2017. In addition to cdsco regulations, the legal metrology packaged commodity. Medical Device Labeling Requirements India.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Medical Device Labeling Requirements India He central drugs standard control organisation(cdsco)under directorate general of health services,ministry of health & family. 89 rows medical device & diagnostics. Alternatively the label shall bear the shelf life of the product. In addition to cdsco regulations, the legal metrology packaged commodity act of 2009 and rules of 2011 is a mandatory license for the manufacture of medical and in.. Medical Device Labeling Requirements India.
From www.schlafenderhase.com
A Guide to Medical Device Labeling Requirements Schlafender Hase Medical Device Labeling Requirements India In addition to cdsco regulations, the legal metrology packaged commodity act of 2009 and rules of 2011 is a mandatory license for the manufacture of medical and in. In india, all medical devices are regulated under the drugs & cosmetics act, 1940 and medical devices rules, 2017 made thereunder. Alternatively the label shall bear the shelf life of the product.. Medical Device Labeling Requirements India.
From www.slideshare.net
Labelling requirements india PPT Medical Device Labeling Requirements India In india, all medical devices are regulated under the drugs & cosmetics act, 1940 and medical devices rules, 2017 made thereunder. Medical device labeling requirements in india are prescribed in chapter vi of the medical devices rules, 2017. 89 rows medical device & diagnostics. In addition to cdsco regulations, the legal metrology packaged commodity act of 2009 and rules of. Medical Device Labeling Requirements India.
From www.slideserve.com
PPT Medical Device Labelling Regulatory Solutions India PowerPoint Medical Device Labeling Requirements India In india, all medical devices are regulated under the drugs & cosmetics act, 1940 and medical devices rules, 2017 made thereunder. Alternatively the label shall bear the shelf life of the product. 89 rows medical device & diagnostics. Medical device manufacturers or importers must provide the labels of the device and ifu, in compliance with chapter vi of labelling of. Medical Device Labeling Requirements India.
From www.slideshare.net
Labelling requirements india PPT Medical Device Labeling Requirements India Discover the essential medical device labeling requirements in india as per the legal metrology act, 2009, and the medical. Medical device manufacturers or importers must provide the labels of the device and ifu, in compliance with chapter vi of labelling of medical devices rules (mdr) 2017. In addition to cdsco regulations, the legal metrology packaged commodity act of 2009 and. Medical Device Labeling Requirements India.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Medical Device Labeling Requirements India In india, all medical devices are regulated under the drugs & cosmetics act, 1940 and medical devices rules, 2017 made thereunder. Discover the essential medical device labeling requirements in india as per the legal metrology act, 2009, and the medical. 89 rows medical device & diagnostics. Medical device manufacturers or importers must provide the labels of the device and ifu,. Medical Device Labeling Requirements India.
From www.vrogue.co
Canadian Device Labeling Requirements Ce Mark Package vrogue.co Medical Device Labeling Requirements India In addition to cdsco regulations, the legal metrology packaged commodity act of 2009 and rules of 2011 is a mandatory license for the manufacture of medical and in. Discover the essential medical device labeling requirements in india as per the legal metrology act, 2009, and the medical. Medical device manufacturers or importers must provide the labels of the device and. Medical Device Labeling Requirements India.
From blog.clevercompliance.io
EU Medical Device Labelling Requirements Clever Compliance Medical Device Labeling Requirements India In addition to cdsco regulations, the legal metrology packaged commodity act of 2009 and rules of 2011 is a mandatory license for the manufacture of medical and in. Alternatively the label shall bear the shelf life of the product. Medical device labeling requirements in india are prescribed in chapter vi of the medical devices rules, 2017. He central drugs standard. Medical Device Labeling Requirements India.
From www.rimsys.io
Quick reference guide global medical device UDI requirements and Medical Device Labeling Requirements India In india, all medical devices are regulated under the drugs & cosmetics act, 1940 and medical devices rules, 2017 made thereunder. In addition to cdsco regulations, the legal metrology packaged commodity act of 2009 and rules of 2011 is a mandatory license for the manufacture of medical and in. Medical device labeling requirements in india are prescribed in chapter vi. Medical Device Labeling Requirements India.
From www.teklynx.com
Why TEKLYNX is the Best Labeling Software for Medical Devices Medical Device Labeling Requirements India In addition to cdsco regulations, the legal metrology packaged commodity act of 2009 and rules of 2011 is a mandatory license for the manufacture of medical and in. Ministry of health & family welfare, government of india has released the medical device rules, 2017, effective from 1 st january, 2018 for. Medical device labeling requirements in india are prescribed in. Medical Device Labeling Requirements India.
From www.vrogue.co
Symbols Commonly Used In Medical Device Packaging And vrogue.co Medical Device Labeling Requirements India Medical device labeling requirements in india are prescribed in chapter vi of the medical devices rules, 2017. Discover the essential medical device labeling requirements in india as per the legal metrology act, 2009, and the medical. Ministry of health & family welfare, government of india has released the medical device rules, 2017, effective from 1 st january, 2018 for. Alternatively. Medical Device Labeling Requirements India.
From www.vrogue.co
Medical Device Labeling Requirements In The Philippin vrogue.co Medical Device Labeling Requirements India 89 rows medical device & diagnostics. Discover the essential medical device labeling requirements in india as per the legal metrology act, 2009, and the medical. In addition to cdsco regulations, the legal metrology packaged commodity act of 2009 and rules of 2011 is a mandatory license for the manufacture of medical and in. Medical device labeling requirements in india are. Medical Device Labeling Requirements India.
From www.techsollifesciences.com
EU MDR & IVDR Medical Device Labelling Requirements Medical Device Labeling Requirements India Alternatively the label shall bear the shelf life of the product. In india, all medical devices are regulated under the drugs & cosmetics act, 1940 and medical devices rules, 2017 made thereunder. 89 rows medical device & diagnostics. Medical device labeling requirements in india are prescribed in chapter vi of the medical devices rules, 2017. He central drugs standard control. Medical Device Labeling Requirements India.
From www.slideshare.net
Labelling requirements india PPT Medical Device Labeling Requirements India Discover the essential medical device labeling requirements in india as per the legal metrology act, 2009, and the medical. Alternatively the label shall bear the shelf life of the product. In india, all medical devices are regulated under the drugs & cosmetics act, 1940 and medical devices rules, 2017 made thereunder. Medical device manufacturers or importers must provide the labels. Medical Device Labeling Requirements India.
From old.sermitsiaq.ag
Medical Device Label Template Medical Device Labeling Requirements India He central drugs standard control organisation(cdsco)under directorate general of health services,ministry of health & family. In india, all medical devices are regulated under the drugs & cosmetics act, 1940 and medical devices rules, 2017 made thereunder. Ministry of health & family welfare, government of india has released the medical device rules, 2017, effective from 1 st january, 2018 for. Medical. Medical Device Labeling Requirements India.
From www.aplyon.com
Medical Device Labeling Procedure Bundle Medical Device Labeling Requirements India 89 rows medical device & diagnostics. Ministry of health & family welfare, government of india has released the medical device rules, 2017, effective from 1 st january, 2018 for. Medical device labeling requirements in india are prescribed in chapter vi of the medical devices rules, 2017. Alternatively the label shall bear the shelf life of the product. Discover the essential. Medical Device Labeling Requirements India.
From mavink.com
Medical Device Labeling Symbols Medical Device Labeling Requirements India Discover the essential medical device labeling requirements in india as per the legal metrology act, 2009, and the medical. 89 rows medical device & diagnostics. He central drugs standard control organisation(cdsco)under directorate general of health services,ministry of health & family. Medical device labeling requirements in india are prescribed in chapter vi of the medical devices rules, 2017. In india, all. Medical Device Labeling Requirements India.
From issuu.com
Medical Device Labeling Requirements VISTAAR by VISTAAR Issuu Medical Device Labeling Requirements India Medical device labeling requirements in india are prescribed in chapter vi of the medical devices rules, 2017. Discover the essential medical device labeling requirements in india as per the legal metrology act, 2009, and the medical. Alternatively the label shall bear the shelf life of the product. Medical device manufacturers or importers must provide the labels of the device and. Medical Device Labeling Requirements India.
From andamanmed.com
Medical device labeling requirements in the Philippines Medical Device Labeling Requirements India In india, all medical devices are regulated under the drugs & cosmetics act, 1940 and medical devices rules, 2017 made thereunder. In addition to cdsco regulations, the legal metrology packaged commodity act of 2009 and rules of 2011 is a mandatory license for the manufacture of medical and in. Discover the essential medical device labeling requirements in india as per. Medical Device Labeling Requirements India.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Labeling Requirements India Discover the essential medical device labeling requirements in india as per the legal metrology act, 2009, and the medical. Alternatively the label shall bear the shelf life of the product. He central drugs standard control organisation(cdsco)under directorate general of health services,ministry of health & family. 89 rows medical device & diagnostics. In india, all medical devices are regulated under the. Medical Device Labeling Requirements India.
From mavink.com
Medical Device Labeling Symbols Medical Device Labeling Requirements India 89 rows medical device & diagnostics. In addition to cdsco regulations, the legal metrology packaged commodity act of 2009 and rules of 2011 is a mandatory license for the manufacture of medical and in. Ministry of health & family welfare, government of india has released the medical device rules, 2017, effective from 1 st january, 2018 for. Discover the essential. Medical Device Labeling Requirements India.
From www.greenlight.guru
Am I Complying with FDA Medical Device Labeling Requirements? Medical Device Labeling Requirements India In addition to cdsco regulations, the legal metrology packaged commodity act of 2009 and rules of 2011 is a mandatory license for the manufacture of medical and in. Alternatively the label shall bear the shelf life of the product. He central drugs standard control organisation(cdsco)under directorate general of health services,ministry of health & family. Medical device manufacturers or importers must. Medical Device Labeling Requirements India.
From fyothzknz.blob.core.windows.net
Nafdac Drug Labelling Regulations at Viola Fudge blog Medical Device Labeling Requirements India Alternatively the label shall bear the shelf life of the product. Ministry of health & family welfare, government of india has released the medical device rules, 2017, effective from 1 st january, 2018 for. He central drugs standard control organisation(cdsco)under directorate general of health services,ministry of health & family. Medical device manufacturers or importers must provide the labels of the. Medical Device Labeling Requirements India.
From www.vrogue.co
Medical Device Labeling Requirements What You Need To vrogue.co Medical Device Labeling Requirements India In addition to cdsco regulations, the legal metrology packaged commodity act of 2009 and rules of 2011 is a mandatory license for the manufacture of medical and in. Discover the essential medical device labeling requirements in india as per the legal metrology act, 2009, and the medical. Medical device labeling requirements in india are prescribed in chapter vi of the. Medical Device Labeling Requirements India.
From www.freseniusmedicalcare.com
MedizinprodukteVerordnung Fresenius Medical Care Medical Device Labeling Requirements India He central drugs standard control organisation(cdsco)under directorate general of health services,ministry of health & family. Alternatively the label shall bear the shelf life of the product. Medical device manufacturers or importers must provide the labels of the device and ifu, in compliance with chapter vi of labelling of medical devices rules (mdr) 2017. 89 rows medical device & diagnostics. In. Medical Device Labeling Requirements India.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Medical Device Labeling Requirements India In addition to cdsco regulations, the legal metrology packaged commodity act of 2009 and rules of 2011 is a mandatory license for the manufacture of medical and in. Alternatively the label shall bear the shelf life of the product. He central drugs standard control organisation(cdsco)under directorate general of health services,ministry of health & family. Discover the essential medical device labeling. Medical Device Labeling Requirements India.
From mungfali.com
Medical Device Labeling Symbols Medical Device Labeling Requirements India Medical device labeling requirements in india are prescribed in chapter vi of the medical devices rules, 2017. In addition to cdsco regulations, the legal metrology packaged commodity act of 2009 and rules of 2011 is a mandatory license for the manufacture of medical and in. 89 rows medical device & diagnostics. Ministry of health & family welfare, government of india. Medical Device Labeling Requirements India.
From clin-r.com
Labels for Medical Devices Clin R Medical Device Labeling Requirements India Discover the essential medical device labeling requirements in india as per the legal metrology act, 2009, and the medical. He central drugs standard control organisation(cdsco)under directorate general of health services,ministry of health & family. 89 rows medical device & diagnostics. In addition to cdsco regulations, the legal metrology packaged commodity act of 2009 and rules of 2011 is a mandatory. Medical Device Labeling Requirements India.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Labeling Requirements India Medical device manufacturers or importers must provide the labels of the device and ifu, in compliance with chapter vi of labelling of medical devices rules (mdr) 2017. In addition to cdsco regulations, the legal metrology packaged commodity act of 2009 and rules of 2011 is a mandatory license for the manufacture of medical and in. He central drugs standard control. Medical Device Labeling Requirements India.