Usp Plate Count Chromatography . The new harmonized standard for chromatography has been approved by the pharmacopeial discussion group (pdg) as. This example shows reporting of usp resolution (hh), ep plate count, and usp s/n (figure 5): The guidelines in usp general chapter provide a clearer definition of permissible adjustments during method transfers that meet. The theoretical number of plates calculated using the four methods are indicated in the table below. Also, peaks with more significant distortion,. Plate count is a theoretical number describing the separation efficiency of a chromatography column. Changes to usp chapter 621 on chromatography go into effect on 1 december 2022. Substances containing radionuclides can be quantified in three ways: (2) by cutting the plates into strips. Specifically, in this tip, we look at the changes to the calculations that affect. Results for n varied even for chromatogram a. The half height multiplier has been changed from 5 to 20 in. In short, it is a measure an eluting compound's bandwidth at the time it elutes from a. (1) directly by moving the plate alongside a suitable counter or vice versa;
from www.chegg.com
The new harmonized standard for chromatography has been approved by the pharmacopeial discussion group (pdg) as. In short, it is a measure an eluting compound's bandwidth at the time it elutes from a. Changes to usp chapter 621 on chromatography go into effect on 1 december 2022. (2) by cutting the plates into strips. The theoretical number of plates calculated using the four methods are indicated in the table below. Specifically, in this tip, we look at the changes to the calculations that affect. (1) directly by moving the plate alongside a suitable counter or vice versa; The half height multiplier has been changed from 5 to 20 in. Plate count is a theoretical number describing the separation efficiency of a chromatography column. The guidelines in usp general chapter provide a clearer definition of permissible adjustments during method transfers that meet.
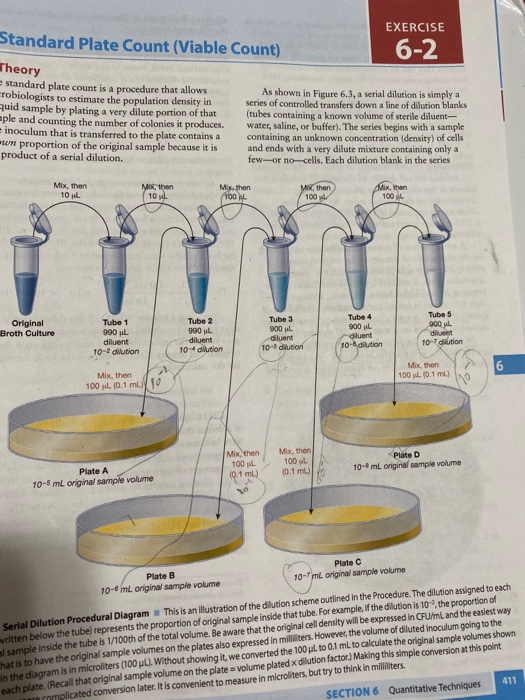
Solved Standard Plate Count (Viable Count) EXERCISE 62
Usp Plate Count Chromatography This example shows reporting of usp resolution (hh), ep plate count, and usp s/n (figure 5): Substances containing radionuclides can be quantified in three ways: The new harmonized standard for chromatography has been approved by the pharmacopeial discussion group (pdg) as. (1) directly by moving the plate alongside a suitable counter or vice versa; Specifically, in this tip, we look at the changes to the calculations that affect. The half height multiplier has been changed from 5 to 20 in. Also, peaks with more significant distortion,. In short, it is a measure an eluting compound's bandwidth at the time it elutes from a. Plate count is a theoretical number describing the separation efficiency of a chromatography column. This example shows reporting of usp resolution (hh), ep plate count, and usp s/n (figure 5): The guidelines in usp general chapter provide a clearer definition of permissible adjustments during method transfers that meet. Changes to usp chapter 621 on chromatography go into effect on 1 december 2022. The theoretical number of plates calculated using the four methods are indicated in the table below. (2) by cutting the plates into strips. Results for n varied even for chromatogram a.
From bio.libretexts.org
Lab 9 Standard Plate Count Biology LibreTexts Usp Plate Count Chromatography The theoretical number of plates calculated using the four methods are indicated in the table below. (1) directly by moving the plate alongside a suitable counter or vice versa; Changes to usp chapter 621 on chromatography go into effect on 1 december 2022. The guidelines in usp general chapter provide a clearer definition of permissible adjustments during method transfers that. Usp Plate Count Chromatography.
From lab-training.com
Concept of Theoretical Plates in Column Chromatography Usp Plate Count Chromatography Also, peaks with more significant distortion,. This example shows reporting of usp resolution (hh), ep plate count, and usp s/n (figure 5): (2) by cutting the plates into strips. The guidelines in usp general chapter provide a clearer definition of permissible adjustments during method transfers that meet. The theoretical number of plates calculated using the four methods are indicated in. Usp Plate Count Chromatography.
From www.youtube.com
Fundamentals of HPLC 24 Calculating Plates YouTube Usp Plate Count Chromatography (1) directly by moving the plate alongside a suitable counter or vice versa; Specifically, in this tip, we look at the changes to the calculations that affect. The guidelines in usp general chapter provide a clearer definition of permissible adjustments during method transfers that meet. The theoretical number of plates calculated using the four methods are indicated in the table. Usp Plate Count Chromatography.
From www.slideserve.com
PPT HPLC Basic Principles and Instrumentation PowerPoint Usp Plate Count Chromatography Specifically, in this tip, we look at the changes to the calculations that affect. Also, peaks with more significant distortion,. The new harmonized standard for chromatography has been approved by the pharmacopeial discussion group (pdg) as. In short, it is a measure an eluting compound's bandwidth at the time it elutes from a. Plate count is a theoretical number describing. Usp Plate Count Chromatography.
From www.coursehero.com
[Solved] Calculate the CFU's from the standard plate count. Course Hero Usp Plate Count Chromatography The guidelines in usp general chapter provide a clearer definition of permissible adjustments during method transfers that meet. Also, peaks with more significant distortion,. Substances containing radionuclides can be quantified in three ways: The theoretical number of plates calculated using the four methods are indicated in the table below. (1) directly by moving the plate alongside a suitable counter or. Usp Plate Count Chromatography.
From www.slideserve.com
PPT Analytical Separations PowerPoint Presentation, free download Usp Plate Count Chromatography Also, peaks with more significant distortion,. (1) directly by moving the plate alongside a suitable counter or vice versa; Changes to usp chapter 621 on chromatography go into effect on 1 december 2022. This example shows reporting of usp resolution (hh), ep plate count, and usp s/n (figure 5): Substances containing radionuclides can be quantified in three ways: Results for. Usp Plate Count Chromatography.
From www.chegg.com
Solved EXERCISE Standard Plate Count (Viable Count) 61 Usp Plate Count Chromatography Changes to usp chapter 621 on chromatography go into effect on 1 december 2022. Also, peaks with more significant distortion,. (1) directly by moving the plate alongside a suitable counter or vice versa; Plate count is a theoretical number describing the separation efficiency of a chromatography column. Results for n varied even for chromatogram a. Substances containing radionuclides can be. Usp Plate Count Chromatography.
From www.youtube.com
Chromatography Efficiency Calculation Simple Method Theoretical Usp Plate Count Chromatography Changes to usp chapter 621 on chromatography go into effect on 1 december 2022. (2) by cutting the plates into strips. Results for n varied even for chromatogram a. The new harmonized standard for chromatography has been approved by the pharmacopeial discussion group (pdg) as. This example shows reporting of usp resolution (hh), ep plate count, and usp s/n (figure. Usp Plate Count Chromatography.
From chem.libretexts.org
12.2 General Theory of Column Chromatography Chemistry LibreTexts Usp Plate Count Chromatography This example shows reporting of usp resolution (hh), ep plate count, and usp s/n (figure 5): The theoretical number of plates calculated using the four methods are indicated in the table below. The new harmonized standard for chromatography has been approved by the pharmacopeial discussion group (pdg) as. Substances containing radionuclides can be quantified in three ways: Changes to usp. Usp Plate Count Chromatography.
From gioxclqfp.blob.core.windows.net
Plate Count Method Usp at Josephine Fletcher blog Usp Plate Count Chromatography Substances containing radionuclides can be quantified in three ways: The guidelines in usp general chapter provide a clearer definition of permissible adjustments during method transfers that meet. Plate count is a theoretical number describing the separation efficiency of a chromatography column. Specifically, in this tip, we look at the changes to the calculations that affect. This example shows reporting of. Usp Plate Count Chromatography.
From www.ijpsonline.com
Optimized Gas ChromatographyMass Spectrometric Method to Profile Usp Plate Count Chromatography The guidelines in usp general chapter provide a clearer definition of permissible adjustments during method transfers that meet. This example shows reporting of usp resolution (hh), ep plate count, and usp s/n (figure 5): (1) directly by moving the plate alongside a suitable counter or vice versa; Also, peaks with more significant distortion,. Specifically, in this tip, we look at. Usp Plate Count Chromatography.
From docplayer.net
ACQUITY UPLC BEH Column Care and Use Instructions PDF Free Download Usp Plate Count Chromatography The guidelines in usp general chapter provide a clearer definition of permissible adjustments during method transfers that meet. The new harmonized standard for chromatography has been approved by the pharmacopeial discussion group (pdg) as. This example shows reporting of usp resolution (hh), ep plate count, and usp s/n (figure 5): (1) directly by moving the plate alongside a suitable counter. Usp Plate Count Chromatography.
From www.slideshare.net
Theories of chromatography Usp Plate Count Chromatography Also, peaks with more significant distortion,. The guidelines in usp general chapter provide a clearer definition of permissible adjustments during method transfers that meet. Specifically, in this tip, we look at the changes to the calculations that affect. This example shows reporting of usp resolution (hh), ep plate count, and usp s/n (figure 5): (2) by cutting the plates into. Usp Plate Count Chromatography.
From owlcation.com
Thin Layer Chromatography (TLC) Principle and Procedure Owlcation Usp Plate Count Chromatography Changes to usp chapter 621 on chromatography go into effect on 1 december 2022. Plate count is a theoretical number describing the separation efficiency of a chromatography column. Also, peaks with more significant distortion,. Specifically, in this tip, we look at the changes to the calculations that affect. (1) directly by moving the plate alongside a suitable counter or vice. Usp Plate Count Chromatography.
From www.slideserve.com
PPT HIGH PERFORMANCE LIQUID CHROMATOGRAPHY (HPLC) PowerPoint Usp Plate Count Chromatography In short, it is a measure an eluting compound's bandwidth at the time it elutes from a. Substances containing radionuclides can be quantified in three ways: Specifically, in this tip, we look at the changes to the calculations that affect. Plate count is a theoretical number describing the separation efficiency of a chromatography column. Results for n varied even for. Usp Plate Count Chromatography.
From www.slideshare.net
USP 621 Allowable Adjustment to Chromatography HPLC Methods Usp Plate Count Chromatography The new harmonized standard for chromatography has been approved by the pharmacopeial discussion group (pdg) as. Specifically, in this tip, we look at the changes to the calculations that affect. (1) directly by moving the plate alongside a suitable counter or vice versa; The guidelines in usp general chapter provide a clearer definition of permissible adjustments during method transfers that. Usp Plate Count Chromatography.
From gioxclqfp.blob.core.windows.net
Plate Count Method Usp at Josephine Fletcher blog Usp Plate Count Chromatography This example shows reporting of usp resolution (hh), ep plate count, and usp s/n (figure 5): Also, peaks with more significant distortion,. Substances containing radionuclides can be quantified in three ways: The half height multiplier has been changed from 5 to 20 in. Specifically, in this tip, we look at the changes to the calculations that affect. In short, it. Usp Plate Count Chromatography.
From halocolumns.com
Options for Optimizing the USP Monograph for Estradiol HALO® Columns Usp Plate Count Chromatography The guidelines in usp general chapter provide a clearer definition of permissible adjustments during method transfers that meet. The half height multiplier has been changed from 5 to 20 in. In short, it is a measure an eluting compound's bandwidth at the time it elutes from a. Results for n varied even for chromatogram a. Also, peaks with more significant. Usp Plate Count Chromatography.
From www.labtestsguide.com
Plate Count Agar (PCA) Purpose, procedure, Composition, Uses and more Usp Plate Count Chromatography The new harmonized standard for chromatography has been approved by the pharmacopeial discussion group (pdg) as. This example shows reporting of usp resolution (hh), ep plate count, and usp s/n (figure 5): The theoretical number of plates calculated using the four methods are indicated in the table below. In short, it is a measure an eluting compound's bandwidth at the. Usp Plate Count Chromatography.
From www.waters.com
Evaluating the Impact of LC System Dispersion on the SizeExclusion Usp Plate Count Chromatography Specifically, in this tip, we look at the changes to the calculations that affect. Substances containing radionuclides can be quantified in three ways: In short, it is a measure an eluting compound's bandwidth at the time it elutes from a. This example shows reporting of usp resolution (hh), ep plate count, and usp s/n (figure 5): Also, peaks with more. Usp Plate Count Chromatography.
From uspharmacopeiaupdate.blogspot.com
Chapter 621 CHROMATOGRAPHY US Pharmacopeia (USP) Usp Plate Count Chromatography Plate count is a theoretical number describing the separation efficiency of a chromatography column. (1) directly by moving the plate alongside a suitable counter or vice versa; Results for n varied even for chromatogram a. (2) by cutting the plates into strips. Also, peaks with more significant distortion,. This example shows reporting of usp resolution (hh), ep plate count, and. Usp Plate Count Chromatography.
From www.researchgate.net
03 Chromatogram oftrial2 Name RT Response Area USP Tailing USP Usp Plate Count Chromatography The guidelines in usp general chapter provide a clearer definition of permissible adjustments during method transfers that meet. (2) by cutting the plates into strips. Changes to usp chapter 621 on chromatography go into effect on 1 december 2022. Substances containing radionuclides can be quantified in three ways: The new harmonized standard for chromatography has been approved by the pharmacopeial. Usp Plate Count Chromatography.
From gpatindia.com
ChromatographyPlate and Rate Theory and MCQ based on GPAT, GATE, UGC Usp Plate Count Chromatography The theoretical number of plates calculated using the four methods are indicated in the table below. Plate count is a theoretical number describing the separation efficiency of a chromatography column. The guidelines in usp general chapter provide a clearer definition of permissible adjustments during method transfers that meet. This example shows reporting of usp resolution (hh), ep plate count, and. Usp Plate Count Chromatography.
From www.chromatographyonline.com
Adjusting HPLC USP Methods Pharmaceutical Analysis Usp Plate Count Chromatography The guidelines in usp general chapter provide a clearer definition of permissible adjustments during method transfers that meet. Substances containing radionuclides can be quantified in three ways: Results for n varied even for chromatogram a. (2) by cutting the plates into strips. Also, peaks with more significant distortion,. This example shows reporting of usp resolution (hh), ep plate count, and. Usp Plate Count Chromatography.
From www.mdpi.com
Separations Free FullText Determination of Sodium, Potassium, and Usp Plate Count Chromatography This example shows reporting of usp resolution (hh), ep plate count, and usp s/n (figure 5): Specifically, in this tip, we look at the changes to the calculations that affect. The guidelines in usp general chapter provide a clearer definition of permissible adjustments during method transfers that meet. Also, peaks with more significant distortion,. (2) by cutting the plates into. Usp Plate Count Chromatography.
From www.researchgate.net
Plate number (or) number of theoretical plates (N) N = 5.545 [t R /w Usp Plate Count Chromatography The guidelines in usp general chapter provide a clearer definition of permissible adjustments during method transfers that meet. (2) by cutting the plates into strips. Specifically, in this tip, we look at the changes to the calculations that affect. In short, it is a measure an eluting compound's bandwidth at the time it elutes from a. Changes to usp chapter. Usp Plate Count Chromatography.
From support.waters.com
Empower Tip of the Week topics covered in 2022 Summary Tip312 Waters Usp Plate Count Chromatography Plate count is a theoretical number describing the separation efficiency of a chromatography column. (1) directly by moving the plate alongside a suitable counter or vice versa; The new harmonized standard for chromatography has been approved by the pharmacopeial discussion group (pdg) as. This example shows reporting of usp resolution (hh), ep plate count, and usp s/n (figure 5): Changes. Usp Plate Count Chromatography.
From www.waters.com
Achieving Method Modernization with the New Liquid Chromatographic Usp Plate Count Chromatography Substances containing radionuclides can be quantified in three ways: This example shows reporting of usp resolution (hh), ep plate count, and usp s/n (figure 5): Also, peaks with more significant distortion,. The half height multiplier has been changed from 5 to 20 in. Results for n varied even for chromatogram a. In short, it is a measure an eluting compound's. Usp Plate Count Chromatography.
From www.chegg.com
Solved Standard Plate Count (Viable Count) EXERCISE 62 Usp Plate Count Chromatography Substances containing radionuclides can be quantified in three ways: (1) directly by moving the plate alongside a suitable counter or vice versa; Specifically, in this tip, we look at the changes to the calculations that affect. This example shows reporting of usp resolution (hh), ep plate count, and usp s/n (figure 5): Changes to usp chapter 621 on chromatography go. Usp Plate Count Chromatography.
From www.youtube.com
Fundamentals of HPLC 26 Plates and Resolution YouTube Usp Plate Count Chromatography The theoretical number of plates calculated using the four methods are indicated in the table below. Changes to usp chapter 621 on chromatography go into effect on 1 december 2022. (2) by cutting the plates into strips. Specifically, in this tip, we look at the changes to the calculations that affect. The new harmonized standard for chromatography has been approved. Usp Plate Count Chromatography.
From www.researchgate.net
Effect tests (Being Nparm = number of parameters associated with this Usp Plate Count Chromatography This example shows reporting of usp resolution (hh), ep plate count, and usp s/n (figure 5): Results for n varied even for chromatogram a. (1) directly by moving the plate alongside a suitable counter or vice versa; Changes to usp chapter 621 on chromatography go into effect on 1 december 2022. The theoretical number of plates calculated using the four. Usp Plate Count Chromatography.
From www.youtube.com
Explain Plate Theory of Chromatography. Chromatography Analytical Usp Plate Count Chromatography (2) by cutting the plates into strips. The half height multiplier has been changed from 5 to 20 in. The new harmonized standard for chromatography has been approved by the pharmacopeial discussion group (pdg) as. Plate count is a theoretical number describing the separation efficiency of a chromatography column. Substances containing radionuclides can be quantified in three ways: The theoretical. Usp Plate Count Chromatography.
From www.youtube.com
Thin Layer Chromatography TLC Principle & Procedure L6 Unit3 Usp Plate Count Chromatography (1) directly by moving the plate alongside a suitable counter or vice versa; The guidelines in usp general chapter provide a clearer definition of permissible adjustments during method transfers that meet. In short, it is a measure an eluting compound's bandwidth at the time it elutes from a. Changes to usp chapter 621 on chromatography go into effect on 1. Usp Plate Count Chromatography.
From innovareacademics.in
The RSD of the peak area of all peaks for five replicate injections Usp Plate Count Chromatography This example shows reporting of usp resolution (hh), ep plate count, and usp s/n (figure 5): The new harmonized standard for chromatography has been approved by the pharmacopeial discussion group (pdg) as. The half height multiplier has been changed from 5 to 20 in. Plate count is a theoretical number describing the separation efficiency of a chromatography column. Substances containing. Usp Plate Count Chromatography.
From www.chromatographyonline.com
Chromatography Fundamentals, Part V Theoretical Plates Significance Usp Plate Count Chromatography Plate count is a theoretical number describing the separation efficiency of a chromatography column. The theoretical number of plates calculated using the four methods are indicated in the table below. The guidelines in usp general chapter provide a clearer definition of permissible adjustments during method transfers that meet. Results for n varied even for chromatogram a. Specifically, in this tip,. Usp Plate Count Chromatography.