What Does Heat Capacity Refer To . It is usually expressed as calories per degree in. The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. It plays a crucial role in understanding. The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Heat capacity is a fundamental concept in thermodynamics and is defined as the amount of heat energy required to raise the. Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a. Heat capacity, ratio of heat absorbed by a material to the temperature change.
from www.youtube.com
Heat capacity is a fundamental concept in thermodynamics and is defined as the amount of heat energy required to raise the. The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a. It is usually expressed as calories per degree in. Heat capacity, ratio of heat absorbed by a material to the temperature change. It plays a crucial role in understanding.
Specific heat capacity YouTube
What Does Heat Capacity Refer To It is usually expressed as calories per degree in. Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Heat capacity, ratio of heat absorbed by a material to the temperature change. Heat capacity is a fundamental concept in thermodynamics and is defined as the amount of heat energy required to raise the. It plays a crucial role in understanding. The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat. The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. It is usually expressed as calories per degree in.
From www.researchgate.net
(a) Measured heat capacity of singlecrystalline Cs 2 TaCl 6 as a What Does Heat Capacity Refer To Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a. It is usually expressed as calories per degree in. Heat capacity, ratio of heat absorbed by a material to the temperature change. The heat capacity of a substance describes how its. What Does Heat Capacity Refer To.
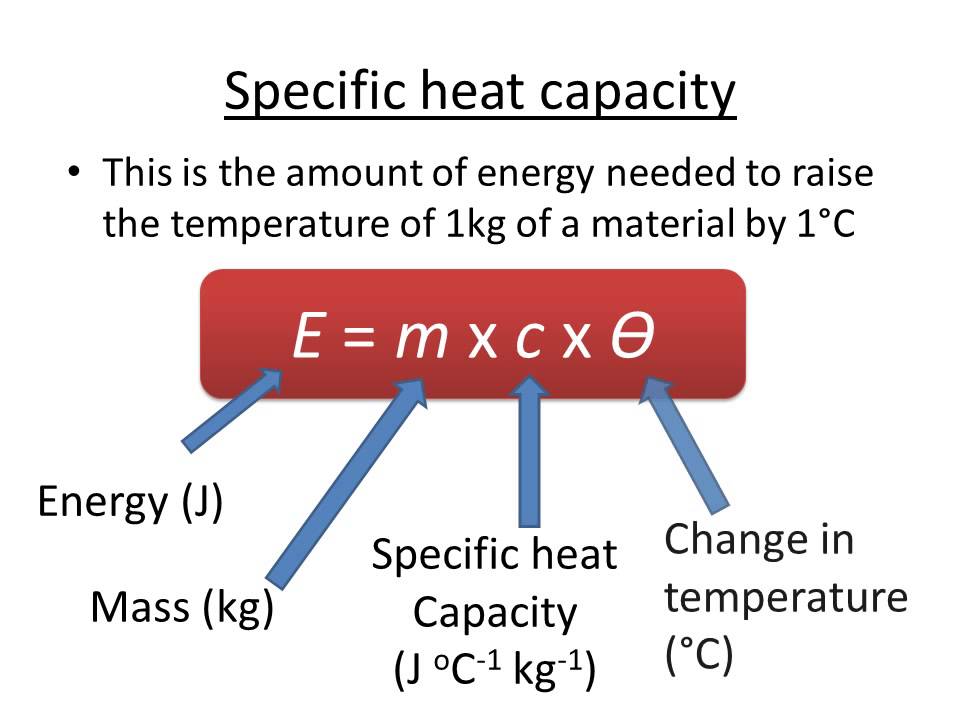
From gbu-presnenskij.ru
Specific Heat Definition Facts Britannica, 47 OFF What Does Heat Capacity Refer To The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. It is usually expressed as calories per degree in. Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a. Heat capacity, ratio. What Does Heat Capacity Refer To.
From www.researchgate.net
Effect of ambient temperatures on heating capacity. Download What Does Heat Capacity Refer To The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. Heat capacity is a fundamental concept in thermodynamics and is defined as the amount of heat energy required to raise the. The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of. What Does Heat Capacity Refer To.
From ch301.cm.utexas.edu
heating curve What Does Heat Capacity Refer To The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat. Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a. It plays a crucial role in. What Does Heat Capacity Refer To.
From www.calameo.com
Calaméo Heat Treatment What Does Heat Capacity Refer To The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Heat capacity, ratio of heat absorbed by a material to. What Does Heat Capacity Refer To.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download What Does Heat Capacity Refer To It plays a crucial role in understanding. The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat. Heat capacity, ratio of heat absorbed by a material to the temperature change. Specific heat is defined as the amount of heat required to raise the temperature of. What Does Heat Capacity Refer To.
From www.youtube.com
Heat Capacity YouTube What Does Heat Capacity Refer To Heat capacity, ratio of heat absorbed by a material to the temperature change. The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat. It plays a crucial role in understanding. Heat capacity is a fundamental concept in thermodynamics and is defined as the amount of. What Does Heat Capacity Refer To.
From the.physicsteachingpodcast.com
Specific Heat Capacity The Physics Teaching Podcast What Does Heat Capacity Refer To Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a. Heat capacity, ratio of heat absorbed by a material to the temperature change. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass. What Does Heat Capacity Refer To.
From studylib.net
Specific Heat Capacity Practice Problems What Does Heat Capacity Refer To Heat capacity, ratio of heat absorbed by a material to the temperature change. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Heat capacity is a fundamental concept in thermodynamics and is defined as the amount of heat energy required to raise the. It. What Does Heat Capacity Refer To.
From calckit.org
CalcKit Specific Heat Capacity Converter What Does Heat Capacity Refer To Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a. The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat. Heat capacity, ratio of heat absorbed. What Does Heat Capacity Refer To.
From www.downwindkennels.com
Specific Heat Capacity What Does Heat Capacity Refer To The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The heat capacity of a substance describes how its temperature changes as it absorbs heat, it. What Does Heat Capacity Refer To.
From jahamattbrown.blogspot.com
Specific Heat Capacity Definition Matt Brown What Does Heat Capacity Refer To It is usually expressed as calories per degree in. Heat capacity, ratio of heat absorbed by a material to the temperature change. The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat. It plays a crucial role in understanding. Specific heat is defined as the. What Does Heat Capacity Refer To.
From sajeewasp.com
Heat capacity and temperature dependence Sajeewa Pemasinghe What Does Heat Capacity Refer To It plays a crucial role in understanding. Heat capacity, ratio of heat absorbed by a material to the temperature change. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The heat capacity of a body is the quantity of heat required to raise its. What Does Heat Capacity Refer To.
From www.savemyexams.com
Determining Specific Heat Capacity WJEC GCSE Physics Revision Notes 2018 What Does Heat Capacity Refer To The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat. Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a. Heat capacity, ratio of heat absorbed. What Does Heat Capacity Refer To.
From www.savemyexams.com
Determining Specific Heat Capacity WJEC GCSE Physics Revision Notes 2018 What Does Heat Capacity Refer To The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat. It is usually expressed as calories per degree in. Specific heat is defined as the amount. What Does Heat Capacity Refer To.
From www.worksheeto.com
12 Best Images of Heat Worksheet 1 Specific Heat Calculations What Does Heat Capacity Refer To Heat capacity, ratio of heat absorbed by a material to the temperature change. The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat. Heat capacity is a fundamental concept in thermodynamics and is defined as the amount of heat energy required to raise the. It. What Does Heat Capacity Refer To.
From www.tec-science.com
Specific heat capacity of selected substances tecscience What Does Heat Capacity Refer To It is usually expressed as calories per degree in. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. Heat capacity, ratio of heat absorbed by. What Does Heat Capacity Refer To.
From studylib.net
Specific Heat Capacity Problems What Does Heat Capacity Refer To Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a. It is usually expressed as calories per degree in. Heat capacity, ratio of heat absorbed by a material to the temperature change. The heat capacity of a substance describes how its. What Does Heat Capacity Refer To.
From classmediaregrouping.z14.web.core.windows.net
Calculating Specific Heat Examples What Does Heat Capacity Refer To Heat capacity, ratio of heat absorbed by a material to the temperature change. It is usually expressed as calories per degree in. Heat capacity is a fundamental concept in thermodynamics and is defined as the amount of heat energy required to raise the. The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is. What Does Heat Capacity Refer To.
From www.greenbuildingadvisor.com
Converting Heat Pump Capacity Between English and Metric What Does Heat Capacity Refer To Heat capacity is a fundamental concept in thermodynamics and is defined as the amount of heat energy required to raise the. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. It plays a crucial role in understanding. Heat capacity (usually denoted by a capital. What Does Heat Capacity Refer To.
From scite.ai
Measurement of Heat Capacity by Adiabatic Calorimetry and Calculation What Does Heat Capacity Refer To It plays a crucial role in understanding. The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat. It is usually expressed as calories per degree in. Heat capacity, ratio of heat absorbed by a material to the temperature change. Heat capacity (usually denoted by a. What Does Heat Capacity Refer To.
From byjus.com
The SI unit of specific heat capacity of a substance is What Does Heat Capacity Refer To It plays a crucial role in understanding. It is usually expressed as calories per degree in. Heat capacity, ratio of heat absorbed by a material to the temperature change. Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a. The heat. What Does Heat Capacity Refer To.
From askfilo.com
The differences between specific heat capacity and heat capacity are.. What Does Heat Capacity Refer To Heat capacity is a fundamental concept in thermodynamics and is defined as the amount of heat energy required to raise the. The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that. What Does Heat Capacity Refer To.
From www.tutorix.com
To determine specific heat capacity of given solid by method of mixtures What Does Heat Capacity Refer To Heat capacity, ratio of heat absorbed by a material to the temperature change. It plays a crucial role in understanding. The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a. What Does Heat Capacity Refer To.
From ar.inspiredpencil.com
Heat Capacity Of Water What Does Heat Capacity Refer To Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Heat capacity is a fundamental concept in thermodynamics and is defined as the amount of heat energy required to raise the. The heat capacity of a substance describes how its temperature changes as it absorbs. What Does Heat Capacity Refer To.
From cuagodep.net
What Is The Molar Heat Capacity Of Water Unraveling Its Thermal Mysteries What Does Heat Capacity Refer To Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a. The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. It plays a crucial role in understanding. Heat capacity, ratio of heat. What Does Heat Capacity Refer To.
From www.youtube.com
Specific heat capacity YouTube What Does Heat Capacity Refer To Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a. The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. Specific heat is defined as the amount of heat required to raise. What Does Heat Capacity Refer To.
From www.researchgate.net
Variations of the isobaric specific heat capacity c p of water vapor What Does Heat Capacity Refer To The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a. Heat capacity is a fundamental concept in thermodynamics and is defined as. What Does Heat Capacity Refer To.
From obropolox.blogspot.com
40 specific heat capacity worksheet answers Worksheet Resource What Does Heat Capacity Refer To The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat. Heat capacity is a fundamental concept in thermodynamics and is defined as the amount of heat energy required to raise the. It is usually expressed as calories per degree in. It plays a crucial role. What Does Heat Capacity Refer To.
From askfilo.com
4. Define heat capacity. What are Cp and Cv ? Show that CP −CV =R. A. T.. What Does Heat Capacity Refer To Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a. Heat capacity, ratio of heat absorbed by a material to the temperature change. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass. What Does Heat Capacity Refer To.
From www.wikidoc.org
Specific heat capacity wikidoc What Does Heat Capacity Refer To The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat. Heat capacity, ratio of heat absorbed by a material to the temperature change. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by. What Does Heat Capacity Refer To.
From authoritysport.blogspot.com
Table Of Specific Heats What Does Heat Capacity Refer To The heat capacity of a body is the quantity of heat required to raise its temperature by one degree. Heat capacity is a fundamental concept in thermodynamics and is defined as the amount of heat energy required to raise the. It plays a crucial role in understanding. It is usually expressed as calories per degree in. The heat capacity of. What Does Heat Capacity Refer To.
From studylib.net
RP 01 Specific Heat Capacity What Does Heat Capacity Refer To The heat capacity of a substance describes how its temperature changes as it absorbs heat, it is the capacity of a substance to absorb heat. Heat capacity is a fundamental concept in thermodynamics and is defined as the amount of heat energy required to raise the. Specific heat is defined as the amount of heat required to raise the temperature. What Does Heat Capacity Refer To.
From ar.inspiredpencil.com
Heat Capacity Chart What Does Heat Capacity Refer To It is usually expressed as calories per degree in. Heat capacity, ratio of heat absorbed by a material to the temperature change. Heat capacity (usually denoted by a capital c, often with subscripts), or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a. The heat capacity of a substance describes how its. What Does Heat Capacity Refer To.
From haipernews.com
How To Calculate How Many Joules Of Heat Haiper What Does Heat Capacity Refer To Heat capacity is a fundamental concept in thermodynamics and is defined as the amount of heat energy required to raise the. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The heat capacity of a body is the quantity of heat required to raise. What Does Heat Capacity Refer To.