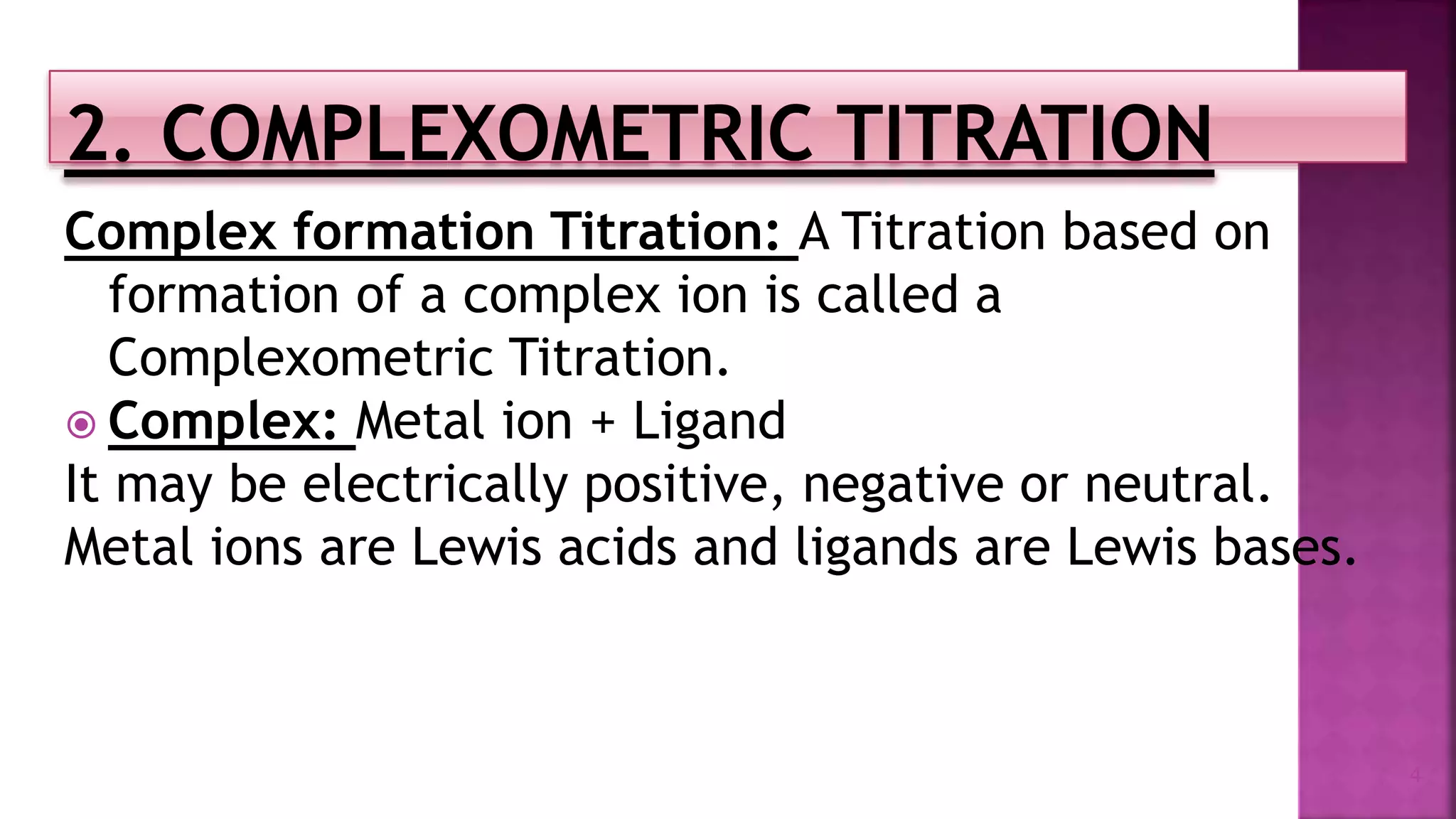
Complexometric Titration Byjus . Complexometric titration is a technique in analytical chemistry that refers to forming stable complexes between metal ions and specific complexing agents. Complexometric titrations mainly depend on the formation of a complex between the analyte and the titrant. Complexometric titration also referred to as chelatometry, is a volumetric analytical technique in which the titration’s endpoint is established using a colored complex. Use the concept of chemical equilibria in complexometric titrations and calculations. Distinguish among the various types of edta. It is greater than the precipitation. Complexometric titration is a volumetric analysis where the endpoint of the analysis or titration is identified by the formation of a coloured. Complexometric titrations the complexometric titration is where an undissociated complex is formed at an equivalence point.
from www.slideshare.net
It is greater than the precipitation. Complexometric titrations the complexometric titration is where an undissociated complex is formed at an equivalence point. Distinguish among the various types of edta. Complexometric titrations mainly depend on the formation of a complex between the analyte and the titrant. Complexometric titration also referred to as chelatometry, is a volumetric analytical technique in which the titration’s endpoint is established using a colored complex. Complexometric titration is a volumetric analysis where the endpoint of the analysis or titration is identified by the formation of a coloured. Complexometric titration is a technique in analytical chemistry that refers to forming stable complexes between metal ions and specific complexing agents. Use the concept of chemical equilibria in complexometric titrations and calculations.
Complexometric Titration
Complexometric Titration Byjus Complexometric titration also referred to as chelatometry, is a volumetric analytical technique in which the titration’s endpoint is established using a colored complex. It is greater than the precipitation. Complexometric titration also referred to as chelatometry, is a volumetric analytical technique in which the titration’s endpoint is established using a colored complex. Complexometric titration is a volumetric analysis where the endpoint of the analysis or titration is identified by the formation of a coloured. Use the concept of chemical equilibria in complexometric titrations and calculations. Distinguish among the various types of edta. Complexometric titrations the complexometric titration is where an undissociated complex is formed at an equivalence point. Complexometric titration is a technique in analytical chemistry that refers to forming stable complexes between metal ions and specific complexing agents. Complexometric titrations mainly depend on the formation of a complex between the analyte and the titrant.
From www.youtube.com
Complexometric Titration Principle and Theory Pharmaceutical Complexometric Titration Byjus Complexometric titration also referred to as chelatometry, is a volumetric analytical technique in which the titration’s endpoint is established using a colored complex. Use the concept of chemical equilibria in complexometric titrations and calculations. It is greater than the precipitation. Complexometric titrations the complexometric titration is where an undissociated complex is formed at an equivalence point. Distinguish among the various. Complexometric Titration Byjus.
From www.scribd.com
KGB Complexometric Titration PDF Coordination Complex Complexometric Titration Byjus Complexometric titration also referred to as chelatometry, is a volumetric analytical technique in which the titration’s endpoint is established using a colored complex. Complexometric titrations the complexometric titration is where an undissociated complex is formed at an equivalence point. It is greater than the precipitation. Complexometric titration is a volumetric analysis where the endpoint of the analysis or titration is. Complexometric Titration Byjus.
From collegedunia.com
Complexometric Titration EDTA, Types & Applications Complexometric Titration Byjus Use the concept of chemical equilibria in complexometric titrations and calculations. Complexometric titrations the complexometric titration is where an undissociated complex is formed at an equivalence point. Complexometric titration also referred to as chelatometry, is a volumetric analytical technique in which the titration’s endpoint is established using a colored complex. Complexometric titrations mainly depend on the formation of a complex. Complexometric Titration Byjus.
From www.youtube.com
COMPLEXOMETRIC TITRATIONS/TITRATIONS/PHARMACEUTICAL ANALYSIS/B PHARM Complexometric Titration Byjus Complexometric titrations mainly depend on the formation of a complex between the analyte and the titrant. Complexometric titration is a volumetric analysis where the endpoint of the analysis or titration is identified by the formation of a coloured. Distinguish among the various types of edta. Use the concept of chemical equilibria in complexometric titrations and calculations. Complexometric titration also referred. Complexometric Titration Byjus.
From www.studypool.com
SOLUTION Complexometric titration principle Studypool Complexometric Titration Byjus Complexometric titrations mainly depend on the formation of a complex between the analyte and the titrant. Complexometric titration also referred to as chelatometry, is a volumetric analytical technique in which the titration’s endpoint is established using a colored complex. It is greater than the precipitation. Distinguish among the various types of edta. Complexometric titration is a volumetric analysis where the. Complexometric Titration Byjus.
From www.studypool.com
SOLUTION Complexometric titration Studypool Complexometric Titration Byjus Complexometric titration also referred to as chelatometry, is a volumetric analytical technique in which the titration’s endpoint is established using a colored complex. Distinguish among the various types of edta. Complexometric titration is a technique in analytical chemistry that refers to forming stable complexes between metal ions and specific complexing agents. Use the concept of chemical equilibria in complexometric titrations. Complexometric Titration Byjus.
From www.slideshare.net
complexometric titration, general chemistry assignment Complexometric Titration Byjus Complexometric titration is a volumetric analysis where the endpoint of the analysis or titration is identified by the formation of a coloured. Distinguish among the various types of edta. It is greater than the precipitation. Complexometric titrations the complexometric titration is where an undissociated complex is formed at an equivalence point. Complexometric titrations mainly depend on the formation of a. Complexometric Titration Byjus.
From www.youtube.com
Complexometric Titration Calculations After Equivalence Point Complexometric Titration Byjus Complexometric titration is a technique in analytical chemistry that refers to forming stable complexes between metal ions and specific complexing agents. Complexometric titration also referred to as chelatometry, is a volumetric analytical technique in which the titration’s endpoint is established using a colored complex. It is greater than the precipitation. Complexometric titrations mainly depend on the formation of a complex. Complexometric Titration Byjus.
From www.youtube.com
Titrations (AcidBase, Redox , Complexometric, Precipitation and Complexometric Titration Byjus It is greater than the precipitation. Complexometric titrations mainly depend on the formation of a complex between the analyte and the titrant. Complexometric titrations the complexometric titration is where an undissociated complex is formed at an equivalence point. Complexometric titration is a technique in analytical chemistry that refers to forming stable complexes between metal ions and specific complexing agents. Complexometric. Complexometric Titration Byjus.
From chem.libretexts.org
Complexation Titration Chemistry LibreTexts Complexometric Titration Byjus Complexometric titration is a technique in analytical chemistry that refers to forming stable complexes between metal ions and specific complexing agents. Complexometric titrations the complexometric titration is where an undissociated complex is formed at an equivalence point. Use the concept of chemical equilibria in complexometric titrations and calculations. Complexometric titrations mainly depend on the formation of a complex between the. Complexometric Titration Byjus.
From www.pharmaacademias.com
Complexometric Titration Pharmaacademias Complexometric Titration Byjus Complexometric titration also referred to as chelatometry, is a volumetric analytical technique in which the titration’s endpoint is established using a colored complex. It is greater than the precipitation. Use the concept of chemical equilibria in complexometric titrations and calculations. Distinguish among the various types of edta. Complexometric titration is a technique in analytical chemistry that refers to forming stable. Complexometric Titration Byjus.
From www.studypool.com
SOLUTION Complexometric titration note Studypool Complexometric Titration Byjus Distinguish among the various types of edta. Complexometric titration also referred to as chelatometry, is a volumetric analytical technique in which the titration’s endpoint is established using a colored complex. Complexometric titrations mainly depend on the formation of a complex between the analyte and the titrant. Use the concept of chemical equilibria in complexometric titrations and calculations. It is greater. Complexometric Titration Byjus.
From www.slideshare.net
Complexometric titrations Complexometric Titration Byjus Complexometric titration is a volumetric analysis where the endpoint of the analysis or titration is identified by the formation of a coloured. Use the concept of chemical equilibria in complexometric titrations and calculations. Complexometric titrations mainly depend on the formation of a complex between the analyte and the titrant. It is greater than the precipitation. Complexometric titration also referred to. Complexometric Titration Byjus.
From www.scribd.com
Tutorial Complexometric Titration PDF Titration Chemistry Complexometric Titration Byjus Complexometric titration is a volumetric analysis where the endpoint of the analysis or titration is identified by the formation of a coloured. Complexometric titrations the complexometric titration is where an undissociated complex is formed at an equivalence point. Distinguish among the various types of edta. Complexometric titration is a technique in analytical chemistry that refers to forming stable complexes between. Complexometric Titration Byjus.
From www.studypool.com
SOLUTION Analytical chemistry complexometric titration q a Studypool Complexometric Titration Byjus Complexometric titrations the complexometric titration is where an undissociated complex is formed at an equivalence point. Use the concept of chemical equilibria in complexometric titrations and calculations. Complexometric titration also referred to as chelatometry, is a volumetric analytical technique in which the titration’s endpoint is established using a colored complex. Complexometric titrations mainly depend on the formation of a complex. Complexometric Titration Byjus.
From www.scribd.com
Lecture 1. Complexometric Titration PDF Coordination Complex Ligand Complexometric Titration Byjus Use the concept of chemical equilibria in complexometric titrations and calculations. Complexometric titrations mainly depend on the formation of a complex between the analyte and the titrant. Complexometric titrations the complexometric titration is where an undissociated complex is formed at an equivalence point. It is greater than the precipitation. Distinguish among the various types of edta. Complexometric titration is a. Complexometric Titration Byjus.
From www.youtube.com
Complexometric titration with EDTAAnimated videoMetal ion indicator Complexometric Titration Byjus Use the concept of chemical equilibria in complexometric titrations and calculations. Complexometric titrations the complexometric titration is where an undissociated complex is formed at an equivalence point. Complexometric titrations mainly depend on the formation of a complex between the analyte and the titrant. Complexometric titration also referred to as chelatometry, is a volumetric analytical technique in which the titration’s endpoint. Complexometric Titration Byjus.
From chemistnotes.com
Complexometric Titration Definition, Types, Indicators, and 5 Reliable Complexometric Titration Byjus Complexometric titrations the complexometric titration is where an undissociated complex is formed at an equivalence point. Complexometric titrations mainly depend on the formation of a complex between the analyte and the titrant. Complexometric titration is a volumetric analysis where the endpoint of the analysis or titration is identified by the formation of a coloured. Complexometric titration also referred to as. Complexometric Titration Byjus.
From www.slideshare.net
Complexometric Titration Complexometric Titration Byjus Complexometric titration is a technique in analytical chemistry that refers to forming stable complexes between metal ions and specific complexing agents. Complexometric titration also referred to as chelatometry, is a volumetric analytical technique in which the titration’s endpoint is established using a colored complex. Distinguish among the various types of edta. It is greater than the precipitation. Complexometric titrations the. Complexometric Titration Byjus.
From www.slideshare.net
Complexometric titration Complexometric Titration Byjus Complexometric titrations mainly depend on the formation of a complex between the analyte and the titrant. Complexometric titrations the complexometric titration is where an undissociated complex is formed at an equivalence point. Use the concept of chemical equilibria in complexometric titrations and calculations. Complexometric titration is a volumetric analysis where the endpoint of the analysis or titration is identified by. Complexometric Titration Byjus.
From www.youtube.com
Part 1 Principle of Complexometric Titration & Complexing Agents YouTube Complexometric Titration Byjus Complexometric titration is a technique in analytical chemistry that refers to forming stable complexes between metal ions and specific complexing agents. Complexometric titrations mainly depend on the formation of a complex between the analyte and the titrant. Complexometric titration also referred to as chelatometry, is a volumetric analytical technique in which the titration’s endpoint is established using a colored complex.. Complexometric Titration Byjus.
From www.chemistrylearner.com
Complexometric Titration Definition, Examples, and Applications Complexometric Titration Byjus Distinguish among the various types of edta. It is greater than the precipitation. Complexometric titrations mainly depend on the formation of a complex between the analyte and the titrant. Use the concept of chemical equilibria in complexometric titrations and calculations. Complexometric titrations the complexometric titration is where an undissociated complex is formed at an equivalence point. Complexometric titration is a. Complexometric Titration Byjus.
From www.slideserve.com
PPT COMPLEXOMETRIC TITRATION PowerPoint Presentation, free download Complexometric Titration Byjus Complexometric titrations the complexometric titration is where an undissociated complex is formed at an equivalence point. Distinguish among the various types of edta. Complexometric titration also referred to as chelatometry, is a volumetric analytical technique in which the titration’s endpoint is established using a colored complex. Use the concept of chemical equilibria in complexometric titrations and calculations. Complexometric titration is. Complexometric Titration Byjus.
From www.studypool.com
SOLUTION Complexometric titration principle Studypool Complexometric Titration Byjus Complexometric titrations the complexometric titration is where an undissociated complex is formed at an equivalence point. Complexometric titration also referred to as chelatometry, is a volumetric analytical technique in which the titration’s endpoint is established using a colored complex. Distinguish among the various types of edta. Use the concept of chemical equilibria in complexometric titrations and calculations. Complexometric titrations mainly. Complexometric Titration Byjus.
From www.slideshare.net
Complexometric titrationppt Complexometric Titration Byjus Use the concept of chemical equilibria in complexometric titrations and calculations. It is greater than the precipitation. Complexometric titrations mainly depend on the formation of a complex between the analyte and the titrant. Distinguish among the various types of edta. Complexometric titration is a technique in analytical chemistry that refers to forming stable complexes between metal ions and specific complexing. Complexometric Titration Byjus.
From www.pharmaacademias.com
Complexometric Titration Pharmaacademias Complexometric Titration Byjus Complexometric titration is a technique in analytical chemistry that refers to forming stable complexes between metal ions and specific complexing agents. Complexometric titrations the complexometric titration is where an undissociated complex is formed at an equivalence point. It is greater than the precipitation. Complexometric titration also referred to as chelatometry, is a volumetric analytical technique in which the titration’s endpoint. Complexometric Titration Byjus.
From slidetodoc.com
Complexometric titrations Complexometry A titration based on the Complexometric Titration Byjus Complexometric titration also referred to as chelatometry, is a volumetric analytical technique in which the titration’s endpoint is established using a colored complex. Complexometric titration is a technique in analytical chemistry that refers to forming stable complexes between metal ions and specific complexing agents. Use the concept of chemical equilibria in complexometric titrations and calculations. Distinguish among the various types. Complexometric Titration Byjus.
From www.youtube.com
Advanced Higher Complexometric Titrations YouTube Complexometric Titration Byjus Use the concept of chemical equilibria in complexometric titrations and calculations. Complexometric titration is a volumetric analysis where the endpoint of the analysis or titration is identified by the formation of a coloured. Complexometric titrations mainly depend on the formation of a complex between the analyte and the titrant. Complexometric titration is a technique in analytical chemistry that refers to. Complexometric Titration Byjus.
From www.youtube.com
Complexometric Titration Calculation of Titration curve Calculation Complexometric Titration Byjus Complexometric titrations mainly depend on the formation of a complex between the analyte and the titrant. Use the concept of chemical equilibria in complexometric titrations and calculations. Complexometric titration also referred to as chelatometry, is a volumetric analytical technique in which the titration’s endpoint is established using a colored complex. Complexometric titration is a technique in analytical chemistry that refers. Complexometric Titration Byjus.
From chrominfo.blogspot.com
Chrominfo Indicators of complexometric titration Complexometric Titration Byjus Complexometric titrations mainly depend on the formation of a complex between the analyte and the titrant. Complexometric titration also referred to as chelatometry, is a volumetric analytical technique in which the titration’s endpoint is established using a colored complex. Distinguish among the various types of edta. Complexometric titration is a technique in analytical chemistry that refers to forming stable complexes. Complexometric Titration Byjus.
From www.slideshare.net
Complexometric titration Complexometric Titration Byjus Complexometric titration is a technique in analytical chemistry that refers to forming stable complexes between metal ions and specific complexing agents. Complexometric titration is a volumetric analysis where the endpoint of the analysis or titration is identified by the formation of a coloured. Complexometric titration also referred to as chelatometry, is a volumetric analytical technique in which the titration’s endpoint. Complexometric Titration Byjus.
From www.scribd.com
Complexometric Titration PDF Chemistry Titration Complexometric Titration Byjus Distinguish among the various types of edta. It is greater than the precipitation. Complexometric titration is a volumetric analysis where the endpoint of the analysis or titration is identified by the formation of a coloured. Complexometric titration is a technique in analytical chemistry that refers to forming stable complexes between metal ions and specific complexing agents. Complexometric titrations the complexometric. Complexometric Titration Byjus.
From www.youtube.com
Methods of Complexometric Titration Direct, Indirect, Replacement B Complexometric Titration Byjus It is greater than the precipitation. Complexometric titration is a volumetric analysis where the endpoint of the analysis or titration is identified by the formation of a coloured. Complexometric titrations mainly depend on the formation of a complex between the analyte and the titrant. Complexometric titrations the complexometric titration is where an undissociated complex is formed at an equivalence point.. Complexometric Titration Byjus.
From www.studypool.com
SOLUTION Complexometric titration principle Studypool Complexometric Titration Byjus Complexometric titrations mainly depend on the formation of a complex between the analyte and the titrant. Complexometric titration also referred to as chelatometry, is a volumetric analytical technique in which the titration’s endpoint is established using a colored complex. It is greater than the precipitation. Complexometric titration is a technique in analytical chemistry that refers to forming stable complexes between. Complexometric Titration Byjus.
From www.slideshare.net
Complexometric titration Complexometric Titration Byjus Complexometric titration is a volumetric analysis where the endpoint of the analysis or titration is identified by the formation of a coloured. Distinguish among the various types of edta. Complexometric titration also referred to as chelatometry, is a volumetric analytical technique in which the titration’s endpoint is established using a colored complex. Complexometric titrations the complexometric titration is where an. Complexometric Titration Byjus.