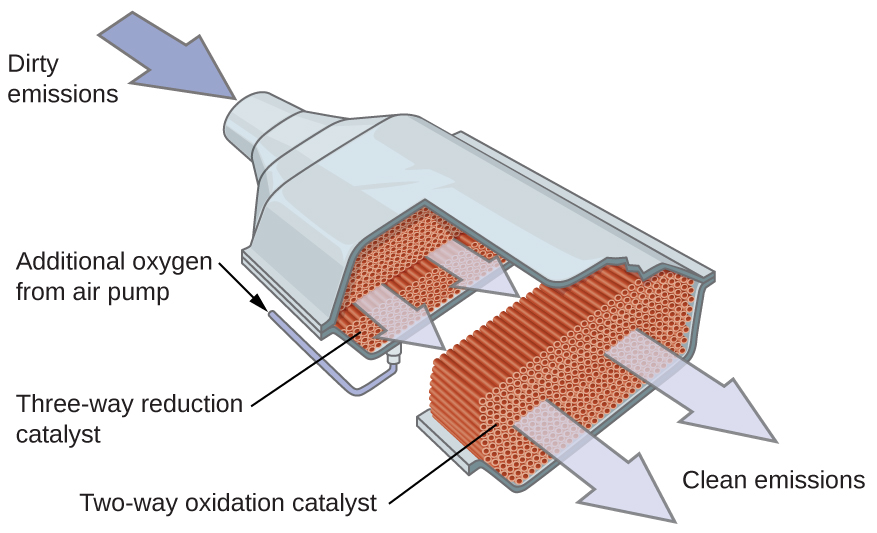
How Does A Catalytic Heater Work . In essence, catalytic heaters only need three things to work: That reaction occurs when propane flows onto the catalytic surface, causing the atomic bonds of the fuel molecules to break down, creating heat as a byproduct to the process. This oxidation process releases heat, which is then radiated outwards, providing the desired warmth. Fuel, oxygen and a catalyst. This eliminates the need for ventilation and reduces safety concerns related to oxygen depletion or releasing harmful gases. In most cases, the fuel comes in the form of either natural gas or propane. How does a catalytic heater work? A catalytic heater is a flameless heater which relies on catalyzed chemical reactions to break down molecules and produce calefaction (heat). Catalytic heaters are flameless heat sources resulting from the catalyzed chemical reactions that occur while breaking down molecules to produce heat, making them ideal for use in. Unlike conventional heaters that rely on a visible flame and consume oxygen, catalytic heaters generate heat through catalysis, ensuring oxygen remains unaffected. The chemical reaction produced in a catalytic heater is fairly simple but highly efficient when it comes to generating heat. In the context of catalytic heaters, the catalyst facilitates the oxidation of fuel, typically propane or natural gas, at temperatures much lower than traditional combustion. A catalytic heater is a flameless heat source that uses chemical reactions to break down molecules and produce heat.
from autotrends.org
The chemical reaction produced in a catalytic heater is fairly simple but highly efficient when it comes to generating heat. That reaction occurs when propane flows onto the catalytic surface, causing the atomic bonds of the fuel molecules to break down, creating heat as a byproduct to the process. Catalytic heaters are flameless heat sources resulting from the catalyzed chemical reactions that occur while breaking down molecules to produce heat, making them ideal for use in. This oxidation process releases heat, which is then radiated outwards, providing the desired warmth. In the context of catalytic heaters, the catalyst facilitates the oxidation of fuel, typically propane or natural gas, at temperatures much lower than traditional combustion. This eliminates the need for ventilation and reduces safety concerns related to oxygen depletion or releasing harmful gases. A catalytic heater is a flameless heat source that uses chemical reactions to break down molecules and produce heat. A catalytic heater is a flameless heater which relies on catalyzed chemical reactions to break down molecules and produce calefaction (heat). In essence, catalytic heaters only need three things to work: Unlike conventional heaters that rely on a visible flame and consume oxygen, catalytic heaters generate heat through catalysis, ensuring oxygen remains unaffected.
The Function and Purpose of a Catalytic Converter Auto Trends Magazine
How Does A Catalytic Heater Work Catalytic heaters are flameless heat sources resulting from the catalyzed chemical reactions that occur while breaking down molecules to produce heat, making them ideal for use in. Unlike conventional heaters that rely on a visible flame and consume oxygen, catalytic heaters generate heat through catalysis, ensuring oxygen remains unaffected. In the context of catalytic heaters, the catalyst facilitates the oxidation of fuel, typically propane or natural gas, at temperatures much lower than traditional combustion. A catalytic heater is a flameless heat source that uses chemical reactions to break down molecules and produce heat. That reaction occurs when propane flows onto the catalytic surface, causing the atomic bonds of the fuel molecules to break down, creating heat as a byproduct to the process. Catalytic heaters are flameless heat sources resulting from the catalyzed chemical reactions that occur while breaking down molecules to produce heat, making them ideal for use in. This oxidation process releases heat, which is then radiated outwards, providing the desired warmth. In most cases, the fuel comes in the form of either natural gas or propane. This eliminates the need for ventilation and reduces safety concerns related to oxygen depletion or releasing harmful gases. How does a catalytic heater work? A catalytic heater is a flameless heater which relies on catalyzed chemical reactions to break down molecules and produce calefaction (heat). Fuel, oxygen and a catalyst. In essence, catalytic heaters only need three things to work: The chemical reaction produced in a catalytic heater is fairly simple but highly efficient when it comes to generating heat.
From www.cjponyparts.com
What Is a Catalytic Converter and How Does It Work? How Does A Catalytic Heater Work The chemical reaction produced in a catalytic heater is fairly simple but highly efficient when it comes to generating heat. This eliminates the need for ventilation and reduces safety concerns related to oxygen depletion or releasing harmful gases. In most cases, the fuel comes in the form of either natural gas or propane. In essence, catalytic heaters only need three. How Does A Catalytic Heater Work.
From trucampers.com
Are Catalytic Heaters Safe in Tents? TruCampers How Does A Catalytic Heater Work Unlike conventional heaters that rely on a visible flame and consume oxygen, catalytic heaters generate heat through catalysis, ensuring oxygen remains unaffected. This eliminates the need for ventilation and reduces safety concerns related to oxygen depletion or releasing harmful gases. How does a catalytic heater work? A catalytic heater is a flameless heater which relies on catalyzed chemical reactions to. How Does A Catalytic Heater Work.
From instagrid.me
The Science Behind Catalytic Converters and Their Role in Reducing How Does A Catalytic Heater Work In most cases, the fuel comes in the form of either natural gas or propane. How does a catalytic heater work? This eliminates the need for ventilation and reduces safety concerns related to oxygen depletion or releasing harmful gases. That reaction occurs when propane flows onto the catalytic surface, causing the atomic bonds of the fuel molecules to break down,. How Does A Catalytic Heater Work.
From autotrends.org
The Function and Purpose of a Catalytic Converter Auto Trends Magazine How Does A Catalytic Heater Work How does a catalytic heater work? This oxidation process releases heat, which is then radiated outwards, providing the desired warmth. A catalytic heater is a flameless heat source that uses chemical reactions to break down molecules and produce heat. The chemical reaction produced in a catalytic heater is fairly simple but highly efficient when it comes to generating heat. This. How Does A Catalytic Heater Work.
From www.hondacrvcatalyticconverter.com
Understanding How A Catalytic Converter Works Honda CRV Catalytic How Does A Catalytic Heater Work In essence, catalytic heaters only need three things to work: How does a catalytic heater work? Fuel, oxygen and a catalyst. Unlike conventional heaters that rely on a visible flame and consume oxygen, catalytic heaters generate heat through catalysis, ensuring oxygen remains unaffected. In most cases, the fuel comes in the form of either natural gas or propane. This oxidation. How Does A Catalytic Heater Work.
From cherokeetulsa.com
Catalytic Heaters Understanding The Science of Safe Heating How Does A Catalytic Heater Work In most cases, the fuel comes in the form of either natural gas or propane. Unlike conventional heaters that rely on a visible flame and consume oxygen, catalytic heaters generate heat through catalysis, ensuring oxygen remains unaffected. This oxidation process releases heat, which is then radiated outwards, providing the desired warmth. A catalytic heater is a flameless heater which relies. How Does A Catalytic Heater Work.
From knowhow.napaonline.com
How A Catalytic Converter Works » NAPA Blog How Does A Catalytic Heater Work In the context of catalytic heaters, the catalyst facilitates the oxidation of fuel, typically propane or natural gas, at temperatures much lower than traditional combustion. This oxidation process releases heat, which is then radiated outwards, providing the desired warmth. That reaction occurs when propane flows onto the catalytic surface, causing the atomic bonds of the fuel molecules to break down,. How Does A Catalytic Heater Work.
From firecatcombustors.blogspot.com
woodstove catalytic combustors Component parts of a wood burning How Does A Catalytic Heater Work Fuel, oxygen and a catalyst. Unlike conventional heaters that rely on a visible flame and consume oxygen, catalytic heaters generate heat through catalysis, ensuring oxygen remains unaffected. This eliminates the need for ventilation and reduces safety concerns related to oxygen depletion or releasing harmful gases. In the context of catalytic heaters, the catalyst facilitates the oxidation of fuel, typically propane. How Does A Catalytic Heater Work.
From cherokeetulsa.com
Catalytic Heaters Understanding The Science of Safe Heating How Does A Catalytic Heater Work Fuel, oxygen and a catalyst. A catalytic heater is a flameless heat source that uses chemical reactions to break down molecules and produce heat. In the context of catalytic heaters, the catalyst facilitates the oxidation of fuel, typically propane or natural gas, at temperatures much lower than traditional combustion. How does a catalytic heater work? The chemical reaction produced in. How Does A Catalytic Heater Work.
From www.goldrattschools.org
How do Catalytic Heaters Work? goldrattschools How Does A Catalytic Heater Work Fuel, oxygen and a catalyst. In the context of catalytic heaters, the catalyst facilitates the oxidation of fuel, typically propane or natural gas, at temperatures much lower than traditional combustion. This eliminates the need for ventilation and reduces safety concerns related to oxygen depletion or releasing harmful gases. How does a catalytic heater work? Catalytic heaters are flameless heat sources. How Does A Catalytic Heater Work.
From www.youtube.com
What is Catalytic Converter? How Catalytic Converter Works? Hindi How Does A Catalytic Heater Work This oxidation process releases heat, which is then radiated outwards, providing the desired warmth. Catalytic heaters are flameless heat sources resulting from the catalyzed chemical reactions that occur while breaking down molecules to produce heat, making them ideal for use in. A catalytic heater is a flameless heater which relies on catalyzed chemical reactions to break down molecules and produce. How Does A Catalytic Heater Work.
From www.fastreplacementglass.com
What Is A Catalytic Combustor & How Does It Work? How Does A Catalytic Heater Work In essence, catalytic heaters only need three things to work: This eliminates the need for ventilation and reduces safety concerns related to oxygen depletion or releasing harmful gases. In most cases, the fuel comes in the form of either natural gas or propane. This oxidation process releases heat, which is then radiated outwards, providing the desired warmth. A catalytic heater. How Does A Catalytic Heater Work.
From www.explainthatstuff.com
How do catalytic converters work? Explain that Stuff How Does A Catalytic Heater Work This eliminates the need for ventilation and reduces safety concerns related to oxygen depletion or releasing harmful gases. A catalytic heater is a flameless heater which relies on catalyzed chemical reactions to break down molecules and produce calefaction (heat). In the context of catalytic heaters, the catalyst facilitates the oxidation of fuel, typically propane or natural gas, at temperatures much. How Does A Catalytic Heater Work.
From themotorguy.com
P0420 Catalyst System Efficiency Below Threshold (Bank 1) The Motor Guy How Does A Catalytic Heater Work In the context of catalytic heaters, the catalyst facilitates the oxidation of fuel, typically propane or natural gas, at temperatures much lower than traditional combustion. That reaction occurs when propane flows onto the catalytic surface, causing the atomic bonds of the fuel molecules to break down, creating heat as a byproduct to the process. The chemical reaction produced in a. How Does A Catalytic Heater Work.
From firecatcombustors.blogspot.com
woodstove catalytic combustors How does the catalytic combustor work? How Does A Catalytic Heater Work Fuel, oxygen and a catalyst. In most cases, the fuel comes in the form of either natural gas or propane. A catalytic heater is a flameless heater which relies on catalyzed chemical reactions to break down molecules and produce calefaction (heat). This eliminates the need for ventilation and reduces safety concerns related to oxygen depletion or releasing harmful gases. The. How Does A Catalytic Heater Work.
From ar.inspiredpencil.com
How A Catalytic Converter Works How Does A Catalytic Heater Work Catalytic heaters are flameless heat sources resulting from the catalyzed chemical reactions that occur while breaking down molecules to produce heat, making them ideal for use in. In most cases, the fuel comes in the form of either natural gas or propane. That reaction occurs when propane flows onto the catalytic surface, causing the atomic bonds of the fuel molecules. How Does A Catalytic Heater Work.
From blog.olx.com.pk
Everything You Need to Know About Catalytic Converters How Does A Catalytic Heater Work How does a catalytic heater work? This eliminates the need for ventilation and reduces safety concerns related to oxygen depletion or releasing harmful gases. In the context of catalytic heaters, the catalyst facilitates the oxidation of fuel, typically propane or natural gas, at temperatures much lower than traditional combustion. The chemical reaction produced in a catalytic heater is fairly simple. How Does A Catalytic Heater Work.
From kimray.com
Catalytic Heater Installation Guide Kimray How Does A Catalytic Heater Work Catalytic heaters are flameless heat sources resulting from the catalyzed chemical reactions that occur while breaking down molecules to produce heat, making them ideal for use in. In most cases, the fuel comes in the form of either natural gas or propane. Fuel, oxygen and a catalyst. How does a catalytic heater work? That reaction occurs when propane flows onto. How Does A Catalytic Heater Work.
From innovationdiscoveries.space
Catalytic Converter (3way) How Does A Catalytic Heater Work This oxidation process releases heat, which is then radiated outwards, providing the desired warmth. Fuel, oxygen and a catalyst. A catalytic heater is a flameless heater which relies on catalyzed chemical reactions to break down molecules and produce calefaction (heat). This eliminates the need for ventilation and reduces safety concerns related to oxygen depletion or releasing harmful gases. A catalytic. How Does A Catalytic Heater Work.
From www.rvingbeginner.com
How Hot Does a Catalytic Converter Get RVing Beginner How Does A Catalytic Heater Work In the context of catalytic heaters, the catalyst facilitates the oxidation of fuel, typically propane or natural gas, at temperatures much lower than traditional combustion. Fuel, oxygen and a catalyst. A catalytic heater is a flameless heat source that uses chemical reactions to break down molecules and produce heat. The chemical reaction produced in a catalytic heater is fairly simple. How Does A Catalytic Heater Work.
From kimray.com
Catalytic Heater Installation Guide Kimray How Does A Catalytic Heater Work This eliminates the need for ventilation and reduces safety concerns related to oxygen depletion or releasing harmful gases. Unlike conventional heaters that rely on a visible flame and consume oxygen, catalytic heaters generate heat through catalysis, ensuring oxygen remains unaffected. This oxidation process releases heat, which is then radiated outwards, providing the desired warmth. Fuel, oxygen and a catalyst. A. How Does A Catalytic Heater Work.
From hdpmotor.blogspot.com
how to work catalytic converter hdp motor How Does A Catalytic Heater Work A catalytic heater is a flameless heat source that uses chemical reactions to break down molecules and produce heat. In most cases, the fuel comes in the form of either natural gas or propane. That reaction occurs when propane flows onto the catalytic surface, causing the atomic bonds of the fuel molecules to break down, creating heat as a byproduct. How Does A Catalytic Heater Work.
From engineeringlearner.com
What is Catalytic Converter? Engineering Learner How Does A Catalytic Heater Work This eliminates the need for ventilation and reduces safety concerns related to oxygen depletion or releasing harmful gases. That reaction occurs when propane flows onto the catalytic surface, causing the atomic bonds of the fuel molecules to break down, creating heat as a byproduct to the process. How does a catalytic heater work? In the context of catalytic heaters, the. How Does A Catalytic Heater Work.
From www.behance.net
Catalytic Converter Vector Illustration Behance How Does A Catalytic Heater Work How does a catalytic heater work? That reaction occurs when propane flows onto the catalytic surface, causing the atomic bonds of the fuel molecules to break down, creating heat as a byproduct to the process. A catalytic heater is a flameless heater which relies on catalyzed chemical reactions to break down molecules and produce calefaction (heat). Unlike conventional heaters that. How Does A Catalytic Heater Work.
From www.merityre.co.uk
Catalytic Converters Merityre Specialists How Does A Catalytic Heater Work In the context of catalytic heaters, the catalyst facilitates the oxidation of fuel, typically propane or natural gas, at temperatures much lower than traditional combustion. Fuel, oxygen and a catalyst. Unlike conventional heaters that rely on a visible flame and consume oxygen, catalytic heaters generate heat through catalysis, ensuring oxygen remains unaffected. The chemical reaction produced in a catalytic heater. How Does A Catalytic Heater Work.
From www.youtube.com
Catalytic Converter Working Principle 2 way and 3 way, Function of How Does A Catalytic Heater Work Fuel, oxygen and a catalyst. The chemical reaction produced in a catalytic heater is fairly simple but highly efficient when it comes to generating heat. In essence, catalytic heaters only need three things to work: How does a catalytic heater work? This oxidation process releases heat, which is then radiated outwards, providing the desired warmth. Catalytic heaters are flameless heat. How Does A Catalytic Heater Work.
From www.rv.com
The Pros and Cons of Using a Catalytic Heater in an RV How Does A Catalytic Heater Work Catalytic heaters are flameless heat sources resulting from the catalyzed chemical reactions that occur while breaking down molecules to produce heat, making them ideal for use in. This eliminates the need for ventilation and reduces safety concerns related to oxygen depletion or releasing harmful gases. That reaction occurs when propane flows onto the catalytic surface, causing the atomic bonds of. How Does A Catalytic Heater Work.
From mycuglobal.org
HOW THE CATALYTIC CONVERTER WORKS AND COSTS My CU Global How Does A Catalytic Heater Work Unlike conventional heaters that rely on a visible flame and consume oxygen, catalytic heaters generate heat through catalysis, ensuring oxygen remains unaffected. In most cases, the fuel comes in the form of either natural gas or propane. Fuel, oxygen and a catalyst. A catalytic heater is a flameless heat source that uses chemical reactions to break down molecules and produce. How Does A Catalytic Heater Work.
From publishthispost.com
What Are Catalytic Heaters and How Do They Work? How Does A Catalytic Heater Work That reaction occurs when propane flows onto the catalytic surface, causing the atomic bonds of the fuel molecules to break down, creating heat as a byproduct to the process. The chemical reaction produced in a catalytic heater is fairly simple but highly efficient when it comes to generating heat. This oxidation process releases heat, which is then radiated outwards, providing. How Does A Catalytic Heater Work.
From www.firecatcombustors.com
FireCatâ„¢ Catalytic Combustors User's Guide How Does A Catalytic Heater Work The chemical reaction produced in a catalytic heater is fairly simple but highly efficient when it comes to generating heat. This eliminates the need for ventilation and reduces safety concerns related to oxygen depletion or releasing harmful gases. Unlike conventional heaters that rely on a visible flame and consume oxygen, catalytic heaters generate heat through catalysis, ensuring oxygen remains unaffected.. How Does A Catalytic Heater Work.
From www.youtube.com
CataDyne How To Start A Catalytic Heater YouTube How Does A Catalytic Heater Work Catalytic heaters are flameless heat sources resulting from the catalyzed chemical reactions that occur while breaking down molecules to produce heat, making them ideal for use in. In most cases, the fuel comes in the form of either natural gas or propane. That reaction occurs when propane flows onto the catalytic surface, causing the atomic bonds of the fuel molecules. How Does A Catalytic Heater Work.
From www.youtube.com
How 3 way catalytic converters work by Howstuffinmycarworks YouTube How Does A Catalytic Heater Work This eliminates the need for ventilation and reduces safety concerns related to oxygen depletion or releasing harmful gases. How does a catalytic heater work? In the context of catalytic heaters, the catalyst facilitates the oxidation of fuel, typically propane or natural gas, at temperatures much lower than traditional combustion. This oxidation process releases heat, which is then radiated outwards, providing. How Does A Catalytic Heater Work.
From firecatcombustors.blogspot.com
woodstove catalytic combustors How a catalytic combustor works. How Does A Catalytic Heater Work Fuel, oxygen and a catalyst. This eliminates the need for ventilation and reduces safety concerns related to oxygen depletion or releasing harmful gases. In essence, catalytic heaters only need three things to work: The chemical reaction produced in a catalytic heater is fairly simple but highly efficient when it comes to generating heat. A catalytic heater is a flameless heater. How Does A Catalytic Heater Work.
From blog.catcousa.com
How Do Catalytic Heaters Work? CATCO How Does A Catalytic Heater Work How does a catalytic heater work? Catalytic heaters are flameless heat sources resulting from the catalyzed chemical reactions that occur while breaking down molecules to produce heat, making them ideal for use in. That reaction occurs when propane flows onto the catalytic surface, causing the atomic bonds of the fuel molecules to break down, creating heat as a byproduct to. How Does A Catalytic Heater Work.
From kimray.com
Catalytic Heater Kimray How Does A Catalytic Heater Work This oxidation process releases heat, which is then radiated outwards, providing the desired warmth. In the context of catalytic heaters, the catalyst facilitates the oxidation of fuel, typically propane or natural gas, at temperatures much lower than traditional combustion. In essence, catalytic heaters only need three things to work: This eliminates the need for ventilation and reduces safety concerns related. How Does A Catalytic Heater Work.