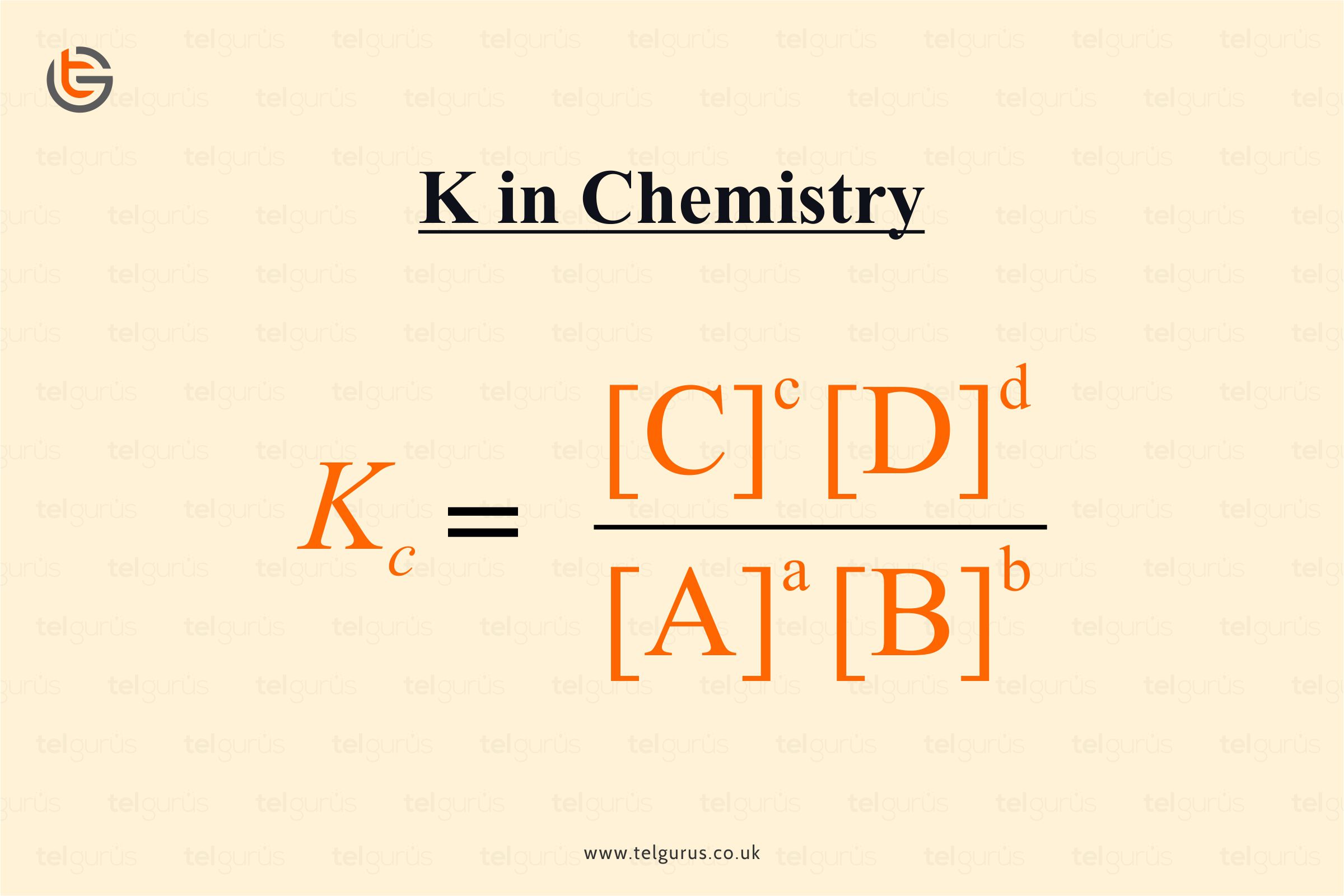
Meaning Of K In Chemistry . The equilibrium constant, k, indicates the extent of a chemical reaction at equilibrium. A small value means that. Most potassium (95 %) goes into fertilizers and the. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. $q$ is computed the same as $k$ except that you plug in the actual concentrations you have, not the values at equilibrium. A large value of the equilibrium constant \(k\) means that products predominate at equilibrium; The chemistry of potassium is almost etirely that of the potassium ion, k +. The equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to a. In more detail, the equilibrium constant, k, is a measure.
from telgurus.co.uk
The chemistry of potassium is almost etirely that of the potassium ion, k +. $q$ is computed the same as $k$ except that you plug in the actual concentrations you have, not the values at equilibrium. The equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to a. Most potassium (95 %) goes into fertilizers and the. A large value of the equilibrium constant \(k\) means that products predominate at equilibrium; In more detail, the equilibrium constant, k, is a measure. The equilibrium constant, k, indicates the extent of a chemical reaction at equilibrium. A small value means that. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and.
Explain the Lock and key mechanism in relation to enzymes. Science
Meaning Of K In Chemistry The equilibrium constant, k, indicates the extent of a chemical reaction at equilibrium. A small value means that. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. A large value of the equilibrium constant \(k\) means that products predominate at equilibrium; The chemistry of potassium is almost etirely that of the potassium ion, k +. The equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to a. $q$ is computed the same as $k$ except that you plug in the actual concentrations you have, not the values at equilibrium. The equilibrium constant, k, indicates the extent of a chemical reaction at equilibrium. In more detail, the equilibrium constant, k, is a measure. Most potassium (95 %) goes into fertilizers and the.
From www.youtube.com
Basics of Chemistry Units of measurement in chemistry YouTube Meaning Of K In Chemistry In more detail, the equilibrium constant, k, is a measure. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. The equilibrium constant, k, indicates the extent of a chemical reaction at equilibrium. The equilibrium constant, k, expresses the relationship between products and reactants of a reaction at. Meaning Of K In Chemistry.
From www.slideserve.com
PPT Chemical Equilibrium CHAPTER 15 Chemistry The Molecular Nature Meaning Of K In Chemistry A large value of the equilibrium constant \(k\) means that products predominate at equilibrium; The equilibrium constant, k, indicates the extent of a chemical reaction at equilibrium. Most potassium (95 %) goes into fertilizers and the. In more detail, the equilibrium constant, k, is a measure. The chemistry of potassium is almost etirely that of the potassium ion, k +.. Meaning Of K In Chemistry.
From www.pinterest.com
K = The Equilibrium Constant. [A] = Molarity of A in the solution Meaning Of K In Chemistry A small value means that. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. In more detail, the equilibrium constant, k, is a measure. Most potassium (95 %) goes into fertilizers and the. $q$ is computed the same as $k$ except that you plug in the actual. Meaning Of K In Chemistry.
From www.slideserve.com
PPT Water Chemistry PowerPoint Presentation, free download ID6639841 Meaning Of K In Chemistry A small value means that. Most potassium (95 %) goes into fertilizers and the. The equilibrium constant, k, indicates the extent of a chemical reaction at equilibrium. The chemistry of potassium is almost etirely that of the potassium ion, k +. The equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to. Meaning Of K In Chemistry.
From nap.nationalacademies.org
2 Precursor Chemicals Used to Make Homemade Explosives Reducing the Meaning Of K In Chemistry The equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to a. $q$ is computed the same as $k$ except that you plug in the actual concentrations you have, not the values at equilibrium. The equilibrium constant, k, indicates the extent of a chemical reaction at equilibrium. A small value means that.. Meaning Of K In Chemistry.
From telgurus.co.uk
Explain the Lock and key mechanism in relation to enzymes. Science Meaning Of K In Chemistry In more detail, the equilibrium constant, k, is a measure. Most potassium (95 %) goes into fertilizers and the. $q$ is computed the same as $k$ except that you plug in the actual concentrations you have, not the values at equilibrium. A large value of the equilibrium constant \(k\) means that products predominate at equilibrium; The chemistry of potassium is. Meaning Of K In Chemistry.
From www.slideshare.net
AP Chemistry Chapter 15 Outline Meaning Of K In Chemistry The equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to a. A large value of the equilibrium constant \(k\) means that products predominate at equilibrium; $q$ is computed the same as $k$ except that you plug in the actual concentrations you have, not the values at equilibrium. Most potassium (95 %). Meaning Of K In Chemistry.
From ar.inspiredpencil.com
Ka Values And Acids Meaning Of K In Chemistry The equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to a. $q$ is computed the same as $k$ except that you plug in the actual concentrations you have, not the values at equilibrium. In more detail, the equilibrium constant, k, is a measure. The chemistry of potassium is almost etirely that. Meaning Of K In Chemistry.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID Meaning Of K In Chemistry The equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to a. In more detail, the equilibrium constant, k, is a measure. Most potassium (95 %) goes into fertilizers and the. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products. Meaning Of K In Chemistry.
From shandenlilah.blogspot.com
22+ Calculate Kb From Ph ShandenLilah Meaning Of K In Chemistry A large value of the equilibrium constant \(k\) means that products predominate at equilibrium; A small value means that. In more detail, the equilibrium constant, k, is a measure. The equilibrium constant, k, indicates the extent of a chemical reaction at equilibrium. The chemistry of potassium is almost etirely that of the potassium ion, k +. $q$ is computed the. Meaning Of K In Chemistry.
From www.youtube.com
Units of k for Zero, 1st, and 2nd Order Reactions YouTube Meaning Of K In Chemistry The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. In more detail, the equilibrium constant, k, is a measure. A small value means that. A large value of the equilibrium constant \(k\) means that products predominate at equilibrium; $q$ is computed the same as $k$ except that. Meaning Of K In Chemistry.
From www.slideserve.com
PPT Ionization Constants of Acids and Bases Strengths of Acids and Meaning Of K In Chemistry A large value of the equilibrium constant \(k\) means that products predominate at equilibrium; $q$ is computed the same as $k$ except that you plug in the actual concentrations you have, not the values at equilibrium. The equilibrium constant, k, indicates the extent of a chemical reaction at equilibrium. The chemistry of potassium is almost etirely that of the potassium. Meaning Of K In Chemistry.
From www.slideserve.com
PPT Chemical Equilibrium CHAPTER 15 Chemistry The Molecular Nature Meaning Of K In Chemistry The equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to a. A small value means that. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. In more detail, the equilibrium constant, k, is a measure. The chemistry of. Meaning Of K In Chemistry.
From www.youtube.com
Chemical Equilibrium Constant K Ice Tables Kp and Kc YouTube Meaning Of K In Chemistry A large value of the equilibrium constant \(k\) means that products predominate at equilibrium; The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. The equilibrium constant, k, indicates the extent of a chemical reaction at equilibrium. Most potassium (95 %) goes into fertilizers and the. $q$ is. Meaning Of K In Chemistry.
From in.pinterest.com
Chemistry alphabet Teaching chemistry, Chemistry, Medical laboratory Meaning Of K In Chemistry The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. The chemistry of potassium is almost etirely that of the potassium ion, k +. Most potassium (95 %) goes into fertilizers and the. In more detail, the equilibrium constant, k, is a measure. The equilibrium constant, k, indicates. Meaning Of K In Chemistry.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Meaning Of K In Chemistry In more detail, the equilibrium constant, k, is a measure. The chemistry of potassium is almost etirely that of the potassium ion, k +. A large value of the equilibrium constant \(k\) means that products predominate at equilibrium; A small value means that. Most potassium (95 %) goes into fertilizers and the. The equilibrium constant, k, indicates the extent of. Meaning Of K In Chemistry.
From www.thoughtco.com
pKa Definition in Chemistry Meaning Of K In Chemistry Most potassium (95 %) goes into fertilizers and the. A small value means that. A large value of the equilibrium constant \(k\) means that products predominate at equilibrium; The chemistry of potassium is almost etirely that of the potassium ion, k +. $q$ is computed the same as $k$ except that you plug in the actual concentrations you have, not. Meaning Of K In Chemistry.
From chemistryskills.com
What Is Kc In Chemistry? Chemistry Skills Meaning Of K In Chemistry A small value means that. $q$ is computed the same as $k$ except that you plug in the actual concentrations you have, not the values at equilibrium. Most potassium (95 %) goes into fertilizers and the. In more detail, the equilibrium constant, k, is a measure. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides. Meaning Of K In Chemistry.
From general.chemistrysteps.com
Reaction Quotient Q Chemistry Steps Meaning Of K In Chemistry The equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to a. $q$ is computed the same as $k$ except that you plug in the actual concentrations you have, not the values at equilibrium. Most potassium (95 %) goes into fertilizers and the. The equilibrium constant, k, indicates the extent of a. Meaning Of K In Chemistry.
From www.studocu.com
Kdefinitions OOOOOOOOOOOOOO Chemistry 100 The Meaning of “K” In Meaning Of K In Chemistry The equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to a. In more detail, the equilibrium constant, k, is a measure. The chemistry of potassium is almost etirely that of the potassium ion, k +. A small value means that. A large value of the equilibrium constant \(k\) means that products. Meaning Of K In Chemistry.
From www.dorjegurung.com
The ABC’s of Chemistry Symbols Meaning Of K In Chemistry A large value of the equilibrium constant \(k\) means that products predominate at equilibrium; The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. The equilibrium constant, k, indicates the extent of a chemical reaction at equilibrium. A small value means that. $q$ is computed the same as. Meaning Of K In Chemistry.
From sciencenotes.org
What Is pKa in Chemistry? Acid Dissociation Constant Meaning Of K In Chemistry Most potassium (95 %) goes into fertilizers and the. The equilibrium constant, k, indicates the extent of a chemical reaction at equilibrium. The chemistry of potassium is almost etirely that of the potassium ion, k +. $q$ is computed the same as $k$ except that you plug in the actual concentrations you have, not the values at equilibrium. In more. Meaning Of K In Chemistry.
From www.slideserve.com
PPT Solubility Equilibria PowerPoint Presentation, free download ID Meaning Of K In Chemistry The equilibrium constant, k, indicates the extent of a chemical reaction at equilibrium. A large value of the equilibrium constant \(k\) means that products predominate at equilibrium; Most potassium (95 %) goes into fertilizers and the. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. The equilibrium. Meaning Of K In Chemistry.
From www.showme.com
Magnitude of Kc Science, Chemistry, Physical Chemistry ShowMe Meaning Of K In Chemistry $q$ is computed the same as $k$ except that you plug in the actual concentrations you have, not the values at equilibrium. In more detail, the equilibrium constant, k, is a measure. The chemistry of potassium is almost etirely that of the potassium ion, k +. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides. Meaning Of K In Chemistry.
From www.showme.com
The Chemists Toolbox The Meaning of K Science, Chemistry ShowMe Meaning Of K In Chemistry The chemistry of potassium is almost etirely that of the potassium ion, k +. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. In more detail, the equilibrium constant, k, is a measure. $q$ is computed the same as $k$ except that you plug in the actual. Meaning Of K In Chemistry.
From 2012books.lardbucket.org
Chemical Equilibrium Meaning Of K In Chemistry $q$ is computed the same as $k$ except that you plug in the actual concentrations you have, not the values at equilibrium. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. A small value means that. The chemistry of potassium is almost etirely that of the potassium. Meaning Of K In Chemistry.
From chem.libretexts.org
Gas Equilibrium Constants Kc And Kp Chemistry LibreTexts Meaning Of K In Chemistry The chemistry of potassium is almost etirely that of the potassium ion, k +. Most potassium (95 %) goes into fertilizers and the. $q$ is computed the same as $k$ except that you plug in the actual concentrations you have, not the values at equilibrium. The equilibrium constant, k, expresses the relationship between products and reactants of a reaction at. Meaning Of K In Chemistry.
From general.chemistrysteps.com
Equilibrium Constant Chemistry Steps Meaning Of K In Chemistry In more detail, the equilibrium constant, k, is a measure. The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between the products and. The equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to a. The equilibrium constant, k, indicates the extent of. Meaning Of K In Chemistry.
From learningschoolscrotums.z14.web.core.windows.net
What Does Aq Indicate Meaning Of K In Chemistry A small value means that. The equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to a. $q$ is computed the same as $k$ except that you plug in the actual concentrations you have, not the values at equilibrium. The equilibrium constant of a chemical reaction (usually denoted by the symbol k). Meaning Of K In Chemistry.
From answerlibbharals.z21.web.core.windows.net
K In Chemistry Stands For Meaning Of K In Chemistry The equilibrium constant, k, indicates the extent of a chemical reaction at equilibrium. Most potassium (95 %) goes into fertilizers and the. A small value means that. The equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to a. A large value of the equilibrium constant \(k\) means that products predominate at. Meaning Of K In Chemistry.
From homedeso.vercel.app
What Is The Periodic Table Of Elements Definition Meaning Of K In Chemistry A small value means that. $q$ is computed the same as $k$ except that you plug in the actual concentrations you have, not the values at equilibrium. A large value of the equilibrium constant \(k\) means that products predominate at equilibrium; The equilibrium constant of a chemical reaction (usually denoted by the symbol k) provides insight into the relationship between. Meaning Of K In Chemistry.
From lincrew.main.jp
KATOMS グローブ lincrew.main.jp Meaning Of K In Chemistry Most potassium (95 %) goes into fertilizers and the. A large value of the equilibrium constant \(k\) means that products predominate at equilibrium; The equilibrium constant, k, indicates the extent of a chemical reaction at equilibrium. In more detail, the equilibrium constant, k, is a measure. The chemistry of potassium is almost etirely that of the potassium ion, k +.. Meaning Of K In Chemistry.
From www.slideserve.com
PPT The Equilibrium Constant, K, and The Reaction Quotient, Q Meaning Of K In Chemistry In more detail, the equilibrium constant, k, is a measure. The chemistry of potassium is almost etirely that of the potassium ion, k +. A small value means that. $q$ is computed the same as $k$ except that you plug in the actual concentrations you have, not the values at equilibrium. The equilibrium constant, k, indicates the extent of a. Meaning Of K In Chemistry.
From pametno21.blogspot.com
K Constant Formula Chemistry pametno Meaning Of K In Chemistry A large value of the equilibrium constant \(k\) means that products predominate at equilibrium; The equilibrium constant, k, indicates the extent of a chemical reaction at equilibrium. A small value means that. The chemistry of potassium is almost etirely that of the potassium ion, k +. Most potassium (95 %) goes into fertilizers and the. $q$ is computed the same. Meaning Of K In Chemistry.
From www.ck12.org
K[a], K[b], pK[a] , and pK[b] Example 4 ( Video ) Chemistry CK12 Meaning Of K In Chemistry Most potassium (95 %) goes into fertilizers and the. The equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium with respect to a. $q$ is computed the same as $k$ except that you plug in the actual concentrations you have, not the values at equilibrium. A large value of the equilibrium constant \(k\) means. Meaning Of K In Chemistry.