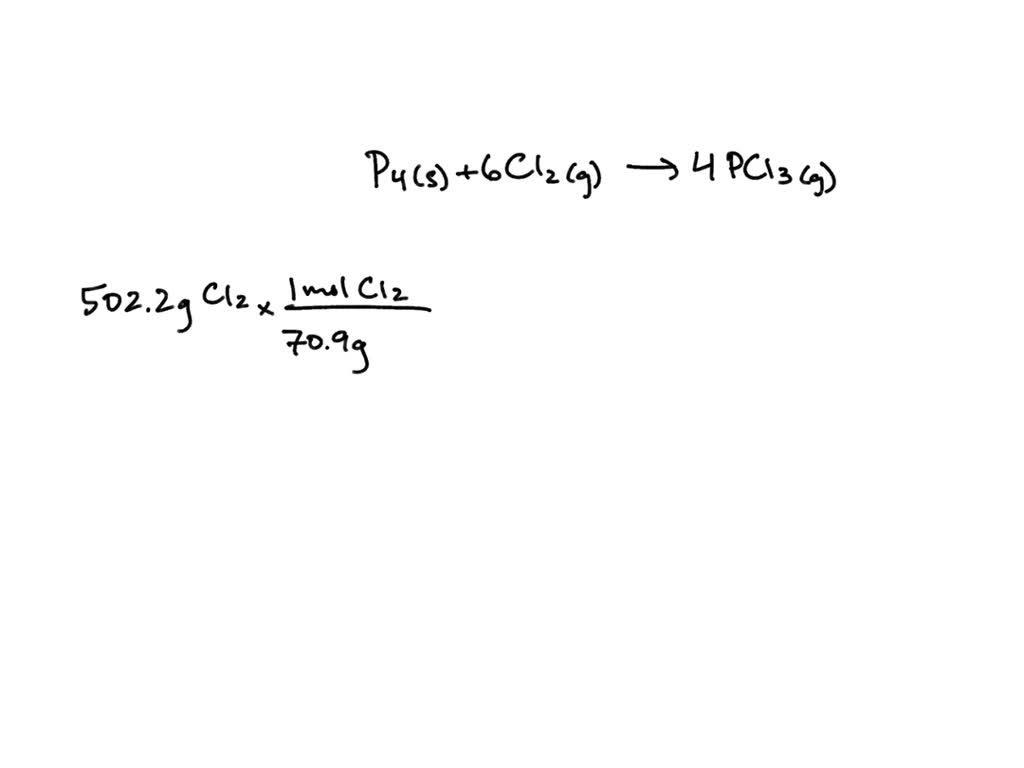
Phosphorus And Chlorine Chemical Formula . Find out what type of reaction occured. At 163°c, the phosphorus(v) chloride converts to a molecular form containing pcl 5 molecules. A chemical equation represents a chemical reaction. Recognize polyatomic ions in chemical formulas. Because only van der waals dispersion forces. It shows the reactants (substances that start a reaction) and products (substances. P4 + cl = pcl3 is a synthesis. P4 + cl2 = pcl3 is a synthesis reaction where one mole of white. Write the chemical formula for a simple ionic compound. The chlorides pcl 3 and pcl 5, both shown in figure 18.41, are the most important halides of phosphorus. Balance any equation or reaction using this chemical equation balancer! White phosphorus + chlorine = phosphorus trichloride. White phosphorus + dichlorine = phosphorus trichloride. We have already encountered some chemical formulas for simple.
from www.numerade.com
The chlorides pcl 3 and pcl 5, both shown in figure 18.41, are the most important halides of phosphorus. It shows the reactants (substances that start a reaction) and products (substances. Balance any equation or reaction using this chemical equation balancer! At 163°c, the phosphorus(v) chloride converts to a molecular form containing pcl 5 molecules. White phosphorus + chlorine = phosphorus trichloride. Write the chemical formula for a simple ionic compound. Recognize polyatomic ions in chemical formulas. Find out what type of reaction occured. P4 + cl = pcl3 is a synthesis. P4 + cl2 = pcl3 is a synthesis reaction where one mole of white.
SOLVED Consider the reaction of solid Pâ‚„ and chlorine gas to form
Phosphorus And Chlorine Chemical Formula Write the chemical formula for a simple ionic compound. We have already encountered some chemical formulas for simple. It shows the reactants (substances that start a reaction) and products (substances. White phosphorus + dichlorine = phosphorus trichloride. The chlorides pcl 3 and pcl 5, both shown in figure 18.41, are the most important halides of phosphorus. At 163°c, the phosphorus(v) chloride converts to a molecular form containing pcl 5 molecules. P4 + cl = pcl3 is a synthesis. Find out what type of reaction occured. A chemical equation represents a chemical reaction. Recognize polyatomic ions in chemical formulas. P4 + cl2 = pcl3 is a synthesis reaction where one mole of white. Write the chemical formula for a simple ionic compound. White phosphorus + chlorine = phosphorus trichloride. Because only van der waals dispersion forces. Balance any equation or reaction using this chemical equation balancer!
From favpng.com
Phosphorus Trifluoride Lewis Structure Trigonal Pyramidal Molecular Phosphorus And Chlorine Chemical Formula White phosphorus + dichlorine = phosphorus trichloride. We have already encountered some chemical formulas for simple. P4 + cl = pcl3 is a synthesis. At 163°c, the phosphorus(v) chloride converts to a molecular form containing pcl 5 molecules. Write the chemical formula for a simple ionic compound. Find out what type of reaction occured. P4 + cl2 = pcl3 is. Phosphorus And Chlorine Chemical Formula.
From giolegnki.blob.core.windows.net
Chlorine Phosphate Chemical Formula at Michael Valenti blog Phosphorus And Chlorine Chemical Formula The chlorides pcl 3 and pcl 5, both shown in figure 18.41, are the most important halides of phosphorus. At 163°c, the phosphorus(v) chloride converts to a molecular form containing pcl 5 molecules. It shows the reactants (substances that start a reaction) and products (substances. P4 + cl = pcl3 is a synthesis. P4 + cl2 = pcl3 is a. Phosphorus And Chlorine Chemical Formula.
From www.chegg.com
Solved A certain compound is made up of one phosphorus (P) Phosphorus And Chlorine Chemical Formula A chemical equation represents a chemical reaction. Recognize polyatomic ions in chemical formulas. Because only van der waals dispersion forces. P4 + cl2 = pcl3 is a synthesis reaction where one mole of white. White phosphorus + chlorine = phosphorus trichloride. At 163°c, the phosphorus(v) chloride converts to a molecular form containing pcl 5 molecules. P4 + cl = pcl3. Phosphorus And Chlorine Chemical Formula.
From www.youtube.com
Balancing and Writing the Equation for Sodium + Chlorine gas YouTube Phosphorus And Chlorine Chemical Formula Recognize polyatomic ions in chemical formulas. At 163°c, the phosphorus(v) chloride converts to a molecular form containing pcl 5 molecules. Because only van der waals dispersion forces. White phosphorus + chlorine = phosphorus trichloride. P4 + cl2 = pcl3 is a synthesis reaction where one mole of white. It shows the reactants (substances that start a reaction) and products (substances.. Phosphorus And Chlorine Chemical Formula.
From www.numerade.com
SOLVED Consider the reaction of solid Pâ‚„ and chlorine gas to form Phosphorus And Chlorine Chemical Formula Balance any equation or reaction using this chemical equation balancer! P4 + cl2 = pcl3 is a synthesis reaction where one mole of white. Recognize polyatomic ions in chemical formulas. We have already encountered some chemical formulas for simple. The chlorides pcl 3 and pcl 5, both shown in figure 18.41, are the most important halides of phosphorus. A chemical. Phosphorus And Chlorine Chemical Formula.
From www.youtube.com
PCl3 Lewis Structure How to Draw the Lewis Structure for PCl3 Phosphorus And Chlorine Chemical Formula Recognize polyatomic ions in chemical formulas. White phosphorus + chlorine = phosphorus trichloride. White phosphorus + dichlorine = phosphorus trichloride. Balance any equation or reaction using this chemical equation balancer! Find out what type of reaction occured. It shows the reactants (substances that start a reaction) and products (substances. P4 + cl = pcl3 is a synthesis. P4 + cl2. Phosphorus And Chlorine Chemical Formula.
From brainly.in
Phosphorus burns in chlorine gas to form phosphorus pentachloride. find Phosphorus And Chlorine Chemical Formula Write the chemical formula for a simple ionic compound. Find out what type of reaction occured. A chemical equation represents a chemical reaction. White phosphorus + chlorine = phosphorus trichloride. Because only van der waals dispersion forces. Balance any equation or reaction using this chemical equation balancer! The chlorides pcl 3 and pcl 5, both shown in figure 18.41, are. Phosphorus And Chlorine Chemical Formula.
From imgbin.com
Phosphorus Trichloride Phosphorus Pentachloride Chemical Compound Phosphorus And Chlorine Chemical Formula It shows the reactants (substances that start a reaction) and products (substances. Write the chemical formula for a simple ionic compound. White phosphorus + chlorine = phosphorus trichloride. We have already encountered some chemical formulas for simple. Because only van der waals dispersion forces. The chlorides pcl 3 and pcl 5, both shown in figure 18.41, are the most important. Phosphorus And Chlorine Chemical Formula.
From www.gbiosciences.com
Potassium Phosphate Monobasic Chemical Formula Phosphorus And Chlorine Chemical Formula The chlorides pcl 3 and pcl 5, both shown in figure 18.41, are the most important halides of phosphorus. Balance any equation or reaction using this chemical equation balancer! Because only van der waals dispersion forces. White phosphorus + dichlorine = phosphorus trichloride. P4 + cl2 = pcl3 is a synthesis reaction where one mole of white. Recognize polyatomic ions. Phosphorus And Chlorine Chemical Formula.
From www.shutterstock.com
Lewis Structure Chlorine Cl Stock Vector (Royalty Free) 2115881024 Phosphorus And Chlorine Chemical Formula It shows the reactants (substances that start a reaction) and products (substances. We have already encountered some chemical formulas for simple. P4 + cl = pcl3 is a synthesis. Recognize polyatomic ions in chemical formulas. A chemical equation represents a chemical reaction. Write the chemical formula for a simple ionic compound. Balance any equation or reaction using this chemical equation. Phosphorus And Chlorine Chemical Formula.
From www.youtube.com
Balance P + Cl2 = PCl5 (Phosphorous and Chlorine Gas) YouTube Phosphorus And Chlorine Chemical Formula White phosphorus + dichlorine = phosphorus trichloride. Find out what type of reaction occured. Write the chemical formula for a simple ionic compound. The chlorides pcl 3 and pcl 5, both shown in figure 18.41, are the most important halides of phosphorus. We have already encountered some chemical formulas for simple. Balance any equation or reaction using this chemical equation. Phosphorus And Chlorine Chemical Formula.
From www.numerade.com
SOLVED Phosphorus trichloride gas is formed by the reaction of solid Phosphorus And Chlorine Chemical Formula A chemical equation represents a chemical reaction. Because only van der waals dispersion forces. At 163°c, the phosphorus(v) chloride converts to a molecular form containing pcl 5 molecules. Balance any equation or reaction using this chemical equation balancer! P4 + cl2 = pcl3 is a synthesis reaction where one mole of white. We have already encountered some chemical formulas for. Phosphorus And Chlorine Chemical Formula.
From www.alamy.com
Phosphorus pentachloride is the chemical compound with the formula PCl5 Phosphorus And Chlorine Chemical Formula White phosphorus + dichlorine = phosphorus trichloride. P4 + cl = pcl3 is a synthesis. Write the chemical formula for a simple ionic compound. Find out what type of reaction occured. Because only van der waals dispersion forces. A chemical equation represents a chemical reaction. We have already encountered some chemical formulas for simple. Balance any equation or reaction using. Phosphorus And Chlorine Chemical Formula.
From stock.adobe.com
Lewis structural formula of chlorine, molecular formula Stock Vector Phosphorus And Chlorine Chemical Formula P4 + cl2 = pcl3 is a synthesis reaction where one mole of white. Balance any equation or reaction using this chemical equation balancer! Write the chemical formula for a simple ionic compound. White phosphorus + chlorine = phosphorus trichloride. We have already encountered some chemical formulas for simple. It shows the reactants (substances that start a reaction) and products. Phosphorus And Chlorine Chemical Formula.
From www.numerade.com
SOLVED Names of molecular compounds Formula Name Formula Name CF5 Phosphorus And Chlorine Chemical Formula Balance any equation or reaction using this chemical equation balancer! Recognize polyatomic ions in chemical formulas. The chlorides pcl 3 and pcl 5, both shown in figure 18.41, are the most important halides of phosphorus. White phosphorus + chlorine = phosphorus trichloride. P4 + cl = pcl3 is a synthesis. Write the chemical formula for a simple ionic compound. It. Phosphorus And Chlorine Chemical Formula.
From www.chegg.com
Solved Chlorine gas reacts with solid phosphorus to produce Phosphorus And Chlorine Chemical Formula Recognize polyatomic ions in chemical formulas. Write the chemical formula for a simple ionic compound. A chemical equation represents a chemical reaction. Find out what type of reaction occured. Balance any equation or reaction using this chemical equation balancer! At 163°c, the phosphorus(v) chloride converts to a molecular form containing pcl 5 molecules. We have already encountered some chemical formulas. Phosphorus And Chlorine Chemical Formula.
From www.dreamstime.com
Chlorine Dioxide, ClO2, Ballandstick Model, Molecular and Chemical Phosphorus And Chlorine Chemical Formula White phosphorus + dichlorine = phosphorus trichloride. Because only van der waals dispersion forces. We have already encountered some chemical formulas for simple. The chlorides pcl 3 and pcl 5, both shown in figure 18.41, are the most important halides of phosphorus. Balance any equation or reaction using this chemical equation balancer! Recognize polyatomic ions in chemical formulas. P4 +. Phosphorus And Chlorine Chemical Formula.
From imgbin.com
Lewis Structure Phosphorus Trichloride Phosphorus Pentachloride Phosphorus And Chlorine Chemical Formula Recognize polyatomic ions in chemical formulas. Because only van der waals dispersion forces. White phosphorus + dichlorine = phosphorus trichloride. A chemical equation represents a chemical reaction. Write the chemical formula for a simple ionic compound. The chlorides pcl 3 and pcl 5, both shown in figure 18.41, are the most important halides of phosphorus. It shows the reactants (substances. Phosphorus And Chlorine Chemical Formula.
From www.alamy.com
Molecular Model of Chlorine (Cl2) Molecule. Vector Illustration Stock Phosphorus And Chlorine Chemical Formula Because only van der waals dispersion forces. White phosphorus + chlorine = phosphorus trichloride. We have already encountered some chemical formulas for simple. P4 + cl = pcl3 is a synthesis. White phosphorus + dichlorine = phosphorus trichloride. Find out what type of reaction occured. Write the chemical formula for a simple ionic compound. P4 + cl2 = pcl3 is. Phosphorus And Chlorine Chemical Formula.
From www.dreamstime.com
Phosphorus Pentachloride Molecule 3d, Molecular Structure, Ball and Phosphorus And Chlorine Chemical Formula Find out what type of reaction occured. A chemical equation represents a chemical reaction. P4 + cl2 = pcl3 is a synthesis reaction where one mole of white. White phosphorus + dichlorine = phosphorus trichloride. It shows the reactants (substances that start a reaction) and products (substances. P4 + cl = pcl3 is a synthesis. Because only van der waals. Phosphorus And Chlorine Chemical Formula.
From www.meritnation.com
Q1 Write the chemical equation for Phosphorus burns in chlorine to form Phosphorus And Chlorine Chemical Formula At 163°c, the phosphorus(v) chloride converts to a molecular form containing pcl 5 molecules. P4 + cl = pcl3 is a synthesis. White phosphorus + dichlorine = phosphorus trichloride. Write the chemical formula for a simple ionic compound. A chemical equation represents a chemical reaction. Find out what type of reaction occured. Because only van der waals dispersion forces. P4. Phosphorus And Chlorine Chemical Formula.
From es.dreamstime.com
Molécula del cloro de Cl2 ilustración del vector. Ilustración de Phosphorus And Chlorine Chemical Formula Find out what type of reaction occured. P4 + cl2 = pcl3 is a synthesis reaction where one mole of white. It shows the reactants (substances that start a reaction) and products (substances. P4 + cl = pcl3 is a synthesis. A chemical equation represents a chemical reaction. Write the chemical formula for a simple ionic compound. At 163°c, the. Phosphorus And Chlorine Chemical Formula.
From www.linstitute.net
AQA A Level Chemistry复习笔记6.1.2 Reaction with Oxygen翰林国际教育 Phosphorus And Chlorine Chemical Formula We have already encountered some chemical formulas for simple. It shows the reactants (substances that start a reaction) and products (substances. P4 + cl = pcl3 is a synthesis. P4 + cl2 = pcl3 is a synthesis reaction where one mole of white. White phosphorus + chlorine = phosphorus trichloride. Because only van der waals dispersion forces. At 163°c, the. Phosphorus And Chlorine Chemical Formula.
From www.fishersci.se
Phosphorus trichloride, 99, Thermo Scientific Chemicals Fisher Phosphorus And Chlorine Chemical Formula A chemical equation represents a chemical reaction. At 163°c, the phosphorus(v) chloride converts to a molecular form containing pcl 5 molecules. The chlorides pcl 3 and pcl 5, both shown in figure 18.41, are the most important halides of phosphorus. Write the chemical formula for a simple ionic compound. Balance any equation or reaction using this chemical equation balancer! Because. Phosphorus And Chlorine Chemical Formula.
From byjus.com
Ine synthesise off phosphorus trichloride by mixing 12 gram of Phosphorus And Chlorine Chemical Formula White phosphorus + chlorine = phosphorus trichloride. It shows the reactants (substances that start a reaction) and products (substances. White phosphorus + dichlorine = phosphorus trichloride. Write the chemical formula for a simple ionic compound. Because only van der waals dispersion forces. P4 + cl = pcl3 is a synthesis. The chlorides pcl 3 and pcl 5, both shown in. Phosphorus And Chlorine Chemical Formula.
From www.dreamstime.com
Phosphate Anion Molecule . Structural Chemical Formula and Mole Stock Phosphorus And Chlorine Chemical Formula The chlorides pcl 3 and pcl 5, both shown in figure 18.41, are the most important halides of phosphorus. White phosphorus + dichlorine = phosphorus trichloride. A chemical equation represents a chemical reaction. Write the chemical formula for a simple ionic compound. At 163°c, the phosphorus(v) chloride converts to a molecular form containing pcl 5 molecules. We have already encountered. Phosphorus And Chlorine Chemical Formula.
From www.numerade.com
SOLVED 'Consider the reaction of solid Pa and chlorine gas to form Phosphorus And Chlorine Chemical Formula White phosphorus + dichlorine = phosphorus trichloride. At 163°c, the phosphorus(v) chloride converts to a molecular form containing pcl 5 molecules. Balance any equation or reaction using this chemical equation balancer! P4 + cl2 = pcl3 is a synthesis reaction where one mole of white. P4 + cl = pcl3 is a synthesis. The chlorides pcl 3 and pcl 5,. Phosphorus And Chlorine Chemical Formula.
From byjus.com
31. How to write a balanced chemical equation when phosphorus reacts Phosphorus And Chlorine Chemical Formula P4 + cl = pcl3 is a synthesis. White phosphorus + chlorine = phosphorus trichloride. Balance any equation or reaction using this chemical equation balancer! White phosphorus + dichlorine = phosphorus trichloride. Find out what type of reaction occured. A chemical equation represents a chemical reaction. P4 + cl2 = pcl3 is a synthesis reaction where one mole of white.. Phosphorus And Chlorine Chemical Formula.
From www.numerade.com
SOLVEDThe reaction of chlorine gas with solid phosphorus (P 4 Phosphorus And Chlorine Chemical Formula The chlorides pcl 3 and pcl 5, both shown in figure 18.41, are the most important halides of phosphorus. A chemical equation represents a chemical reaction. Recognize polyatomic ions in chemical formulas. Find out what type of reaction occured. It shows the reactants (substances that start a reaction) and products (substances. P4 + cl = pcl3 is a synthesis. Because. Phosphorus And Chlorine Chemical Formula.
From www.numerade.com
SOLVED Balance the following equation phosphorous pentachlorde Phosphorus And Chlorine Chemical Formula We have already encountered some chemical formulas for simple. P4 + cl = pcl3 is a synthesis. Find out what type of reaction occured. A chemical equation represents a chemical reaction. Balance any equation or reaction using this chemical equation balancer! White phosphorus + dichlorine = phosphorus trichloride. It shows the reactants (substances that start a reaction) and products (substances.. Phosphorus And Chlorine Chemical Formula.
From www.sarchemlabs.com
Phosphorus Trichloride FAQs Uses, Organic Compounds & More SarChem Labs Phosphorus And Chlorine Chemical Formula A chemical equation represents a chemical reaction. White phosphorus + dichlorine = phosphorus trichloride. We have already encountered some chemical formulas for simple. White phosphorus + chlorine = phosphorus trichloride. At 163°c, the phosphorus(v) chloride converts to a molecular form containing pcl 5 molecules. It shows the reactants (substances that start a reaction) and products (substances. The chlorides pcl 3. Phosphorus And Chlorine Chemical Formula.
From rex-has-melendez.blogspot.com
What Is the Formula for Phosphorus Pentachloride RexhasMelendez Phosphorus And Chlorine Chemical Formula P4 + cl2 = pcl3 is a synthesis reaction where one mole of white. Because only van der waals dispersion forces. It shows the reactants (substances that start a reaction) and products (substances. A chemical equation represents a chemical reaction. Balance any equation or reaction using this chemical equation balancer! P4 + cl = pcl3 is a synthesis. We have. Phosphorus And Chlorine Chemical Formula.
From www.tessshebaylo.com
Salt Water Electrolysis Equation Tessshebaylo Phosphorus And Chlorine Chemical Formula White phosphorus + dichlorine = phosphorus trichloride. White phosphorus + chlorine = phosphorus trichloride. We have already encountered some chemical formulas for simple. It shows the reactants (substances that start a reaction) and products (substances. Because only van der waals dispersion forces. Write the chemical formula for a simple ionic compound. Find out what type of reaction occured. P4 +. Phosphorus And Chlorine Chemical Formula.
From www.pinterest.com
Phosphorus trichloride PCl₃ Molecular Geometry Hybridization Phosphorus And Chlorine Chemical Formula P4 + cl2 = pcl3 is a synthesis reaction where one mole of white. P4 + cl = pcl3 is a synthesis. At 163°c, the phosphorus(v) chloride converts to a molecular form containing pcl 5 molecules. It shows the reactants (substances that start a reaction) and products (substances. Find out what type of reaction occured. Because only van der waals. Phosphorus And Chlorine Chemical Formula.
From schematron.org
Lewis Dot Diagram Phosphorus Wiring Diagram Pictures Phosphorus And Chlorine Chemical Formula Because only van der waals dispersion forces. Balance any equation or reaction using this chemical equation balancer! It shows the reactants (substances that start a reaction) and products (substances. At 163°c, the phosphorus(v) chloride converts to a molecular form containing pcl 5 molecules. Recognize polyatomic ions in chemical formulas. Write the chemical formula for a simple ionic compound. The chlorides. Phosphorus And Chlorine Chemical Formula.