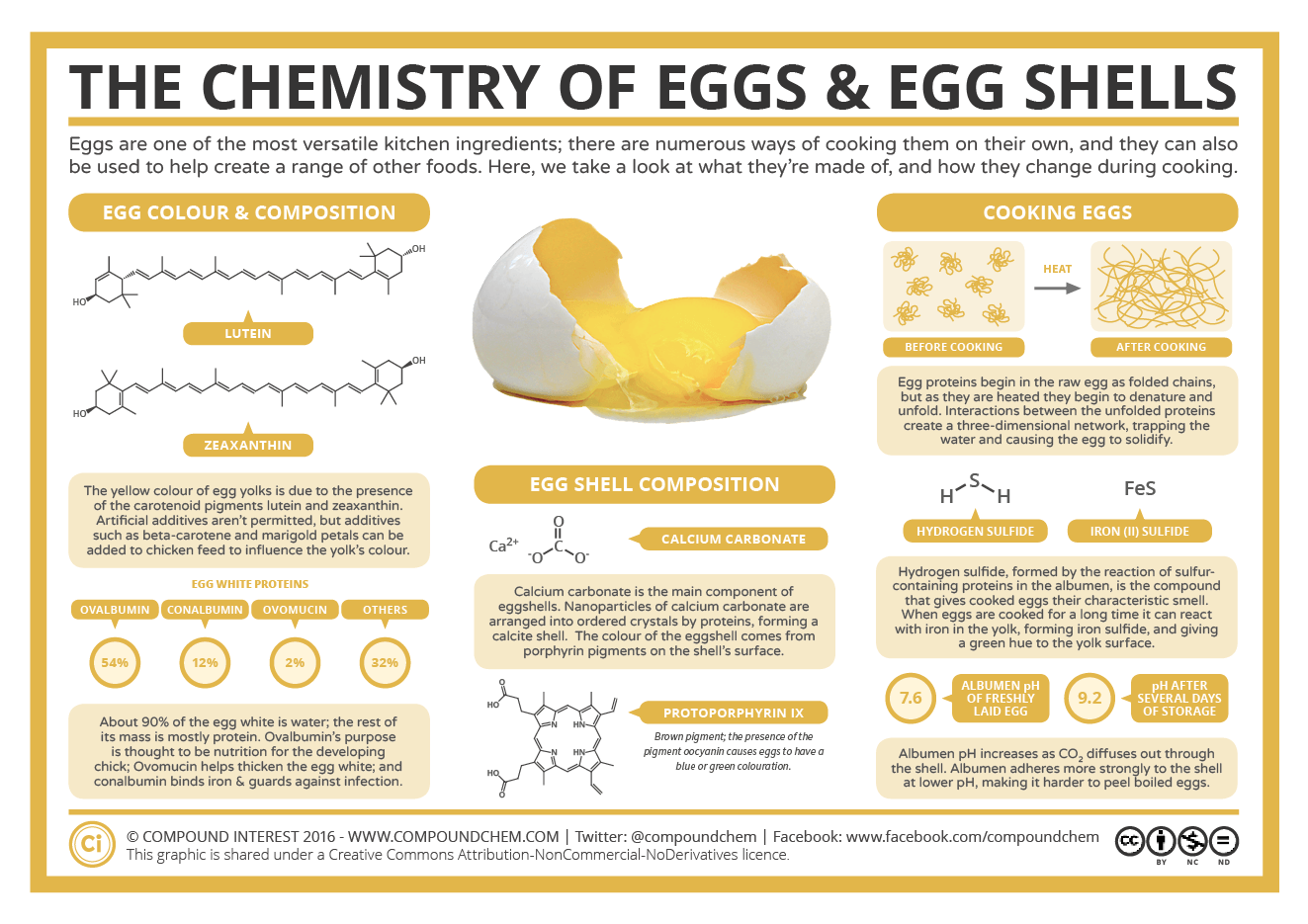
Crushed Egg Shells Reaction With Hcl Equation . Explain the action of dilute hydrochloric acid on the following with chemical equation : Crushed egg shell has composition c a c o 3 reacts with acid and gives gas c o 2 which turns lime water (c a (o h) 2) milky by producing c a. The reaction between eggshell and hydrochloric acid can be. The solution contains (a) nacl (b) hcl (c) licl. Explain the action of the dilute hydrochloric acid on the following with chemical reaction: (c) write the balanced chemical equation of the reaction taking place: Naoh +hcl→ nacl +h 2 o (iii) crushed egg shell are made up of caco 3 which reacts with hcl to give brisk effervescence due. Let us see the reaction of calcium carbonate or egg shells with hydrochloric acid. So, the solution must be hydrochloric acid or $hcl$.
from giodekplp.blob.core.windows.net
Naoh +hcl→ nacl +h 2 o (iii) crushed egg shell are made up of caco 3 which reacts with hcl to give brisk effervescence due. Let us see the reaction of calcium carbonate or egg shells with hydrochloric acid. Explain the action of dilute hydrochloric acid on the following with chemical equation : (c) write the balanced chemical equation of the reaction taking place: Explain the action of the dilute hydrochloric acid on the following with chemical reaction: So, the solution must be hydrochloric acid or $hcl$. The solution contains (a) nacl (b) hcl (c) licl. Crushed egg shell has composition c a c o 3 reacts with acid and gives gas c o 2 which turns lime water (c a (o h) 2) milky by producing c a. The reaction between eggshell and hydrochloric acid can be.
Crushed Egg Shells + Hcl at Johnny Hinman blog
Crushed Egg Shells Reaction With Hcl Equation Explain the action of the dilute hydrochloric acid on the following with chemical reaction: (c) write the balanced chemical equation of the reaction taking place: Naoh +hcl→ nacl +h 2 o (iii) crushed egg shell are made up of caco 3 which reacts with hcl to give brisk effervescence due. The reaction between eggshell and hydrochloric acid can be. Explain the action of dilute hydrochloric acid on the following with chemical equation : The solution contains (a) nacl (b) hcl (c) licl. Explain the action of the dilute hydrochloric acid on the following with chemical reaction: Crushed egg shell has composition c a c o 3 reacts with acid and gives gas c o 2 which turns lime water (c a (o h) 2) milky by producing c a. Let us see the reaction of calcium carbonate or egg shells with hydrochloric acid. So, the solution must be hydrochloric acid or $hcl$.
From brainly.in
explain the action of dilute hydrochloric acid on the following with Crushed Egg Shells Reaction With Hcl Equation Explain the action of the dilute hydrochloric acid on the following with chemical reaction: The reaction between eggshell and hydrochloric acid can be. Crushed egg shell has composition c a c o 3 reacts with acid and gives gas c o 2 which turns lime water (c a (o h) 2) milky by producing c a. (c) write the balanced. Crushed Egg Shells Reaction With Hcl Equation.
From giodekplp.blob.core.windows.net
Crushed Egg Shells + Hcl at Johnny Hinman blog Crushed Egg Shells Reaction With Hcl Equation (c) write the balanced chemical equation of the reaction taking place: Explain the action of dilute hydrochloric acid on the following with chemical equation : The reaction between eggshell and hydrochloric acid can be. So, the solution must be hydrochloric acid or $hcl$. Crushed egg shell has composition c a c o 3 reacts with acid and gives gas c. Crushed Egg Shells Reaction With Hcl Equation.
From exoitmkaw.blob.core.windows.net
Crushed Egg Shells For Weed Plants at William Heinz blog Crushed Egg Shells Reaction With Hcl Equation Crushed egg shell has composition c a c o 3 reacts with acid and gives gas c o 2 which turns lime water (c a (o h) 2) milky by producing c a. Naoh +hcl→ nacl +h 2 o (iii) crushed egg shell are made up of caco 3 which reacts with hcl to give brisk effervescence due. Let us. Crushed Egg Shells Reaction With Hcl Equation.
From www.numerade.com
SOLVED A solution reacts with crushed egg shells to give a gas that Crushed Egg Shells Reaction With Hcl Equation Naoh +hcl→ nacl +h 2 o (iii) crushed egg shell are made up of caco 3 which reacts with hcl to give brisk effervescence due. Let us see the reaction of calcium carbonate or egg shells with hydrochloric acid. Explain the action of the dilute hydrochloric acid on the following with chemical reaction: The solution contains (a) nacl (b) hcl. Crushed Egg Shells Reaction With Hcl Equation.
From www.youtube.com
A solution reacts with crushed eggshells to give a gas that turns lime Crushed Egg Shells Reaction With Hcl Equation (c) write the balanced chemical equation of the reaction taking place: Crushed egg shell has composition c a c o 3 reacts with acid and gives gas c o 2 which turns lime water (c a (o h) 2) milky by producing c a. So, the solution must be hydrochloric acid or $hcl$. The reaction between eggshell and hydrochloric acid. Crushed Egg Shells Reaction With Hcl Equation.
From www.youtube.com
Egg Shell Dissolving Using HCL And Water YouTube Crushed Egg Shells Reaction With Hcl Equation The solution contains (a) nacl (b) hcl (c) licl. Naoh +hcl→ nacl +h 2 o (iii) crushed egg shell are made up of caco 3 which reacts with hcl to give brisk effervescence due. (c) write the balanced chemical equation of the reaction taking place: The reaction between eggshell and hydrochloric acid can be. Crushed egg shell has composition c. Crushed Egg Shells Reaction With Hcl Equation.
From www.gkseries.com
A solution reacts with crushed eggshells to give a gas that turns lime Crushed Egg Shells Reaction With Hcl Equation Explain the action of the dilute hydrochloric acid on the following with chemical reaction: Let us see the reaction of calcium carbonate or egg shells with hydrochloric acid. Crushed egg shell has composition c a c o 3 reacts with acid and gives gas c o 2 which turns lime water (c a (o h) 2) milky by producing c. Crushed Egg Shells Reaction With Hcl Equation.
From exoitmkaw.blob.core.windows.net
Crushed Egg Shells For Weed Plants at William Heinz blog Crushed Egg Shells Reaction With Hcl Equation (c) write the balanced chemical equation of the reaction taking place: Let us see the reaction of calcium carbonate or egg shells with hydrochloric acid. The reaction between eggshell and hydrochloric acid can be. Naoh +hcl→ nacl +h 2 o (iii) crushed egg shell are made up of caco 3 which reacts with hcl to give brisk effervescence due. Crushed. Crushed Egg Shells Reaction With Hcl Equation.
From www.youtube.com
Dissolving EGG in HCl Just Chemistry YouTube Crushed Egg Shells Reaction With Hcl Equation So, the solution must be hydrochloric acid or $hcl$. The reaction between eggshell and hydrochloric acid can be. Explain the action of dilute hydrochloric acid on the following with chemical equation : Let us see the reaction of calcium carbonate or egg shells with hydrochloric acid. Naoh +hcl→ nacl +h 2 o (iii) crushed egg shell are made up of. Crushed Egg Shells Reaction With Hcl Equation.
From exoviutwi.blob.core.windows.net
Crushed Egg Shells Dogs at Maria Kratochvil blog Crushed Egg Shells Reaction With Hcl Equation Explain the action of the dilute hydrochloric acid on the following with chemical reaction: Explain the action of dilute hydrochloric acid on the following with chemical equation : The solution contains (a) nacl (b) hcl (c) licl. So, the solution must be hydrochloric acid or $hcl$. Crushed egg shell has composition c a c o 3 reacts with acid and. Crushed Egg Shells Reaction With Hcl Equation.
From www.youtube.com
Fastest Way to Crush Egg Shells YouTube Crushed Egg Shells Reaction With Hcl Equation Crushed egg shell has composition c a c o 3 reacts with acid and gives gas c o 2 which turns lime water (c a (o h) 2) milky by producing c a. So, the solution must be hydrochloric acid or $hcl$. Naoh +hcl→ nacl +h 2 o (iii) crushed egg shell are made up of caco 3 which reacts. Crushed Egg Shells Reaction With Hcl Equation.
From brainly.in
What happens when dulite HCl reacts with crushed egg shell.Give the Crushed Egg Shells Reaction With Hcl Equation Crushed egg shell has composition c a c o 3 reacts with acid and gives gas c o 2 which turns lime water (c a (o h) 2) milky by producing c a. Let us see the reaction of calcium carbonate or egg shells with hydrochloric acid. Explain the action of the dilute hydrochloric acid on the following with chemical. Crushed Egg Shells Reaction With Hcl Equation.
From www.meritnation.com
Q students dropped few peices of marbles and crushed egg shells in two Crushed Egg Shells Reaction With Hcl Equation (c) write the balanced chemical equation of the reaction taking place: Crushed egg shell has composition c a c o 3 reacts with acid and gives gas c o 2 which turns lime water (c a (o h) 2) milky by producing c a. Explain the action of dilute hydrochloric acid on the following with chemical equation : The reaction. Crushed Egg Shells Reaction With Hcl Equation.
From www.numerade.com
SOLVED a) In an experiment to determine the ( m / m) of calcium Crushed Egg Shells Reaction With Hcl Equation Explain the action of the dilute hydrochloric acid on the following with chemical reaction: Crushed egg shell has composition c a c o 3 reacts with acid and gives gas c o 2 which turns lime water (c a (o h) 2) milky by producing c a. Naoh +hcl→ nacl +h 2 o (iii) crushed egg shell are made up. Crushed Egg Shells Reaction With Hcl Equation.
From www.toppr.com
How the following substance will dissociate to produce ions in their Crushed Egg Shells Reaction With Hcl Equation Naoh +hcl→ nacl +h 2 o (iii) crushed egg shell are made up of caco 3 which reacts with hcl to give brisk effervescence due. Explain the action of the dilute hydrochloric acid on the following with chemical reaction: Explain the action of dilute hydrochloric acid on the following with chemical equation : The solution contains (a) nacl (b) hcl. Crushed Egg Shells Reaction With Hcl Equation.
From brainly.in
when and Excel is reacted with dilute hydrochloric acid, forming is Crushed Egg Shells Reaction With Hcl Equation Crushed egg shell has composition c a c o 3 reacts with acid and gives gas c o 2 which turns lime water (c a (o h) 2) milky by producing c a. So, the solution must be hydrochloric acid or $hcl$. Let us see the reaction of calcium carbonate or egg shells with hydrochloric acid. Explain the action of. Crushed Egg Shells Reaction With Hcl Equation.
From www.alamy.com
A very close view of crushed egg shells Stock Photo Alamy Crushed Egg Shells Reaction With Hcl Equation So, the solution must be hydrochloric acid or $hcl$. Let us see the reaction of calcium carbonate or egg shells with hydrochloric acid. Explain the action of the dilute hydrochloric acid on the following with chemical reaction: Crushed egg shell has composition c a c o 3 reacts with acid and gives gas c o 2 which turns lime water. Crushed Egg Shells Reaction With Hcl Equation.
From www.dreamstime.com
2 Eggs on Bed of Crushed Egg Shell Stock Photo Image of colorful Crushed Egg Shells Reaction With Hcl Equation Let us see the reaction of calcium carbonate or egg shells with hydrochloric acid. (c) write the balanced chemical equation of the reaction taking place: Explain the action of the dilute hydrochloric acid on the following with chemical reaction: Crushed egg shell has composition c a c o 3 reacts with acid and gives gas c o 2 which turns. Crushed Egg Shells Reaction With Hcl Equation.
From exoviutwi.blob.core.windows.net
Crushed Egg Shells Dogs at Maria Kratochvil blog Crushed Egg Shells Reaction With Hcl Equation The reaction between eggshell and hydrochloric acid can be. Crushed egg shell has composition c a c o 3 reacts with acid and gives gas c o 2 which turns lime water (c a (o h) 2) milky by producing c a. So, the solution must be hydrochloric acid or $hcl$. Let us see the reaction of calcium carbonate or. Crushed Egg Shells Reaction With Hcl Equation.
From brainly.in
what happens whens HNO3 is added to egg shell give the reaction Crushed Egg Shells Reaction With Hcl Equation (c) write the balanced chemical equation of the reaction taking place: So, the solution must be hydrochloric acid or $hcl$. Explain the action of the dilute hydrochloric acid on the following with chemical reaction: Explain the action of dilute hydrochloric acid on the following with chemical equation : Let us see the reaction of calcium carbonate or egg shells with. Crushed Egg Shells Reaction With Hcl Equation.
From askfilo.com
A solution reacts with crushed eggshells to give a gas that turns limew.. Crushed Egg Shells Reaction With Hcl Equation So, the solution must be hydrochloric acid or $hcl$. Naoh +hcl→ nacl +h 2 o (iii) crushed egg shell are made up of caco 3 which reacts with hcl to give brisk effervescence due. Crushed egg shell has composition c a c o 3 reacts with acid and gives gas c o 2 which turns lime water (c a (o. Crushed Egg Shells Reaction With Hcl Equation.
From www.youtube.com
What reacts with crushed egg shell to produce a gas that turns lime Crushed Egg Shells Reaction With Hcl Equation Naoh +hcl→ nacl +h 2 o (iii) crushed egg shell are made up of caco 3 which reacts with hcl to give brisk effervescence due. (c) write the balanced chemical equation of the reaction taking place: Explain the action of dilute hydrochloric acid on the following with chemical equation : Let us see the reaction of calcium carbonate or egg. Crushed Egg Shells Reaction With Hcl Equation.
From www.doubtnut.com
Write balanced chemical equation for the following 1. Action of Crushed Egg Shells Reaction With Hcl Equation (c) write the balanced chemical equation of the reaction taking place: Crushed egg shell has composition c a c o 3 reacts with acid and gives gas c o 2 which turns lime water (c a (o h) 2) milky by producing c a. The reaction between eggshell and hydrochloric acid can be. Explain the action of the dilute hydrochloric. Crushed Egg Shells Reaction With Hcl Equation.
From www.toppr.com
Write the balanced chemical equations for the following reactions and Crushed Egg Shells Reaction With Hcl Equation Crushed egg shell has composition c a c o 3 reacts with acid and gives gas c o 2 which turns lime water (c a (o h) 2) milky by producing c a. Naoh +hcl→ nacl +h 2 o (iii) crushed egg shell are made up of caco 3 which reacts with hcl to give brisk effervescence due. Explain the. Crushed Egg Shells Reaction With Hcl Equation.
From www.fresheggsdaily.blog
Crushed Eggshells for your Chickens Fresh Eggs Daily® with Lisa Steele Crushed Egg Shells Reaction With Hcl Equation The solution contains (a) nacl (b) hcl (c) licl. So, the solution must be hydrochloric acid or $hcl$. Naoh +hcl→ nacl +h 2 o (iii) crushed egg shell are made up of caco 3 which reacts with hcl to give brisk effervescence due. Crushed egg shell has composition c a c o 3 reacts with acid and gives gas c. Crushed Egg Shells Reaction With Hcl Equation.
From slideplayer.com
Determine the calcium carbonate content of a sample of an egg shell Crushed Egg Shells Reaction With Hcl Equation Explain the action of dilute hydrochloric acid on the following with chemical equation : (c) write the balanced chemical equation of the reaction taking place: Naoh +hcl→ nacl +h 2 o (iii) crushed egg shell are made up of caco 3 which reacts with hcl to give brisk effervescence due. The reaction between eggshell and hydrochloric acid can be. Crushed. Crushed Egg Shells Reaction With Hcl Equation.
From slideplayer.com
Determine the calcium carbonate content of a sample of an egg shell Crushed Egg Shells Reaction With Hcl Equation Crushed egg shell has composition c a c o 3 reacts with acid and gives gas c o 2 which turns lime water (c a (o h) 2) milky by producing c a. Explain the action of dilute hydrochloric acid on the following with chemical equation : The reaction between eggshell and hydrochloric acid can be. Naoh +hcl→ nacl +h. Crushed Egg Shells Reaction With Hcl Equation.
From www.youtube.com
Magnesium reacting with Hydrochloric Acid YouTube Crushed Egg Shells Reaction With Hcl Equation Explain the action of dilute hydrochloric acid on the following with chemical equation : So, the solution must be hydrochloric acid or $hcl$. Crushed egg shell has composition c a c o 3 reacts with acid and gives gas c o 2 which turns lime water (c a (o h) 2) milky by producing c a. The reaction between eggshell. Crushed Egg Shells Reaction With Hcl Equation.
From brainly.in
egg shells dropped in hydrochloric acid reaction with chemical equation Crushed Egg Shells Reaction With Hcl Equation Explain the action of the dilute hydrochloric acid on the following with chemical reaction: The solution contains (a) nacl (b) hcl (c) licl. The reaction between eggshell and hydrochloric acid can be. (c) write the balanced chemical equation of the reaction taking place: Explain the action of dilute hydrochloric acid on the following with chemical equation : Let us see. Crushed Egg Shells Reaction With Hcl Equation.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Crushed Egg Shells Reaction With Hcl Equation Explain the action of the dilute hydrochloric acid on the following with chemical reaction: The solution contains (a) nacl (b) hcl (c) licl. So, the solution must be hydrochloric acid or $hcl$. The reaction between eggshell and hydrochloric acid can be. Let us see the reaction of calcium carbonate or egg shells with hydrochloric acid. Crushed egg shell has composition. Crushed Egg Shells Reaction With Hcl Equation.
From www.youtube.com
How to Write the Net Ionic Equation for HCl + Ca(OH)2 = CaCl2 + H2O Crushed Egg Shells Reaction With Hcl Equation The solution contains (a) nacl (b) hcl (c) licl. Let us see the reaction of calcium carbonate or egg shells with hydrochloric acid. Naoh +hcl→ nacl +h 2 o (iii) crushed egg shell are made up of caco 3 which reacts with hcl to give brisk effervescence due. So, the solution must be hydrochloric acid or $hcl$. Explain the action. Crushed Egg Shells Reaction With Hcl Equation.
From www.alamy.com
Crushed egg shells Stock Photo Alamy Crushed Egg Shells Reaction With Hcl Equation Explain the action of the dilute hydrochloric acid on the following with chemical reaction: Explain the action of dilute hydrochloric acid on the following with chemical equation : Crushed egg shell has composition c a c o 3 reacts with acid and gives gas c o 2 which turns lime water (c a (o h) 2) milky by producing c. Crushed Egg Shells Reaction With Hcl Equation.
From slideplayer.com
Determine the calcium carbonate content of a sample of an egg shell Crushed Egg Shells Reaction With Hcl Equation Naoh +hcl→ nacl +h 2 o (iii) crushed egg shell are made up of caco 3 which reacts with hcl to give brisk effervescence due. Explain the action of the dilute hydrochloric acid on the following with chemical reaction: Crushed egg shell has composition c a c o 3 reacts with acid and gives gas c o 2 which turns. Crushed Egg Shells Reaction With Hcl Equation.
From www.youtube.com
117 reaction with znco3 and hcl egg shell react with hcl YouTube Crushed Egg Shells Reaction With Hcl Equation Crushed egg shell has composition c a c o 3 reacts with acid and gives gas c o 2 which turns lime water (c a (o h) 2) milky by producing c a. So, the solution must be hydrochloric acid or $hcl$. Explain the action of dilute hydrochloric acid on the following with chemical equation : The solution contains (a). Crushed Egg Shells Reaction With Hcl Equation.
From exoitmkaw.blob.core.windows.net
Crushed Egg Shells For Weed Plants at William Heinz blog Crushed Egg Shells Reaction With Hcl Equation Naoh +hcl→ nacl +h 2 o (iii) crushed egg shell are made up of caco 3 which reacts with hcl to give brisk effervescence due. Let us see the reaction of calcium carbonate or egg shells with hydrochloric acid. So, the solution must be hydrochloric acid or $hcl$. The solution contains (a) nacl (b) hcl (c) licl. (c) write the. Crushed Egg Shells Reaction With Hcl Equation.