Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers . As sodium hydroxide is added it reacts with the acetic acid forming its conjugate base, the salt sodium acetate. To find the concentration of sodium hydroxide by titrating hydrochloric acid with sodium hydroxide. Usually, sodium hydroxide solutions are prepared by diluting a 50% aqueous solution of sodium hydroxide to approximately the desired. Titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. Initially the ph is due to pure acetic acid. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. Titration of hydrochloric acid with sodium hydroxide. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Includes kit list and safety instructions.
from webapi.bu.edu
Titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. Initially the ph is due to pure acetic acid. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. Usually, sodium hydroxide solutions are prepared by diluting a 50% aqueous solution of sodium hydroxide to approximately the desired. As sodium hydroxide is added it reacts with the acetic acid forming its conjugate base, the salt sodium acetate. To find the concentration of sodium hydroxide by titrating hydrochloric acid with sodium hydroxide. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Titration of hydrochloric acid with sodium hydroxide. Includes kit list and safety instructions.
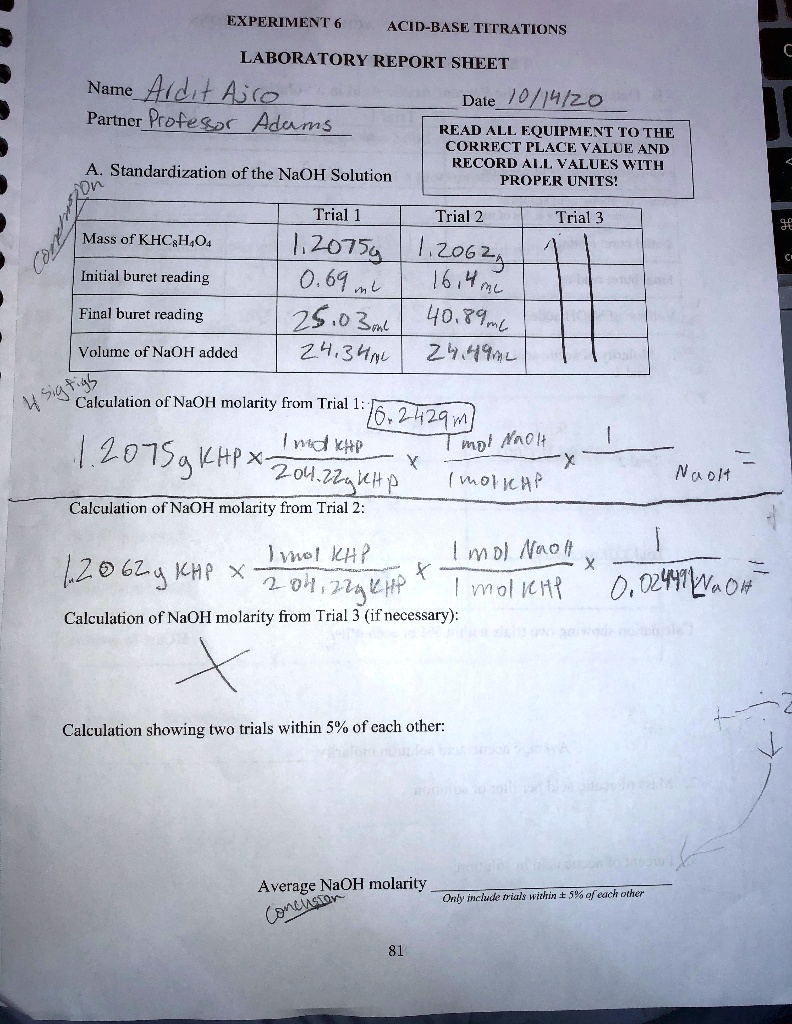
Standardizing a solution of sodium hydroxide lab report answers. Lab
Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers Includes kit list and safety instructions. Titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Titration of hydrochloric acid with sodium hydroxide. As sodium hydroxide is added it reacts with the acetic acid forming its conjugate base, the salt sodium acetate. Usually, sodium hydroxide solutions are prepared by diluting a 50% aqueous solution of sodium hydroxide to approximately the desired. Initially the ph is due to pure acetic acid. To find the concentration of sodium hydroxide by titrating hydrochloric acid with sodium hydroxide. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. Includes kit list and safety instructions.
From frudgereport294.web.fc2.com
Analyze a solution of potassium hydroxide using standard hydrochloric Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers Initially the ph is due to pure acetic acid. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Includes kit list and safety instructions. To find the concentration of sodium hydroxide by titrating hydrochloric acid with sodium hydroxide. As sodium hydroxide is added it reacts with the acetic acid forming its. Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers.
From www.chegg.com
Solved REPORT SHEET EXPERIMENT 20 Titration of Acids and Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers Initially the ph is due to pure acetic acid. Usually, sodium hydroxide solutions are prepared by diluting a 50% aqueous solution of sodium hydroxide to approximately the desired. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. As sodium hydroxide is added it reacts with the acetic acid forming its conjugate. Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers.
From www.scribd.com
Standardization of Hydrochloric Acid Titration Hydrochloric Acid Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers Titration of hydrochloric acid with sodium hydroxide. Titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. To find the concentration of sodium hydroxide by titrating hydrochloric acid with sodium hydroxide. Initially the ph is due to pure acetic acid. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. As. Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers.
From www.numerade.com
SOLVED It takes 21.491 mL to reach the endpoint. For a titration of 25 Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Initially the ph is due to pure acetic acid. Usually, sodium hydroxide solutions are prepared by diluting a 50% aqueous solution of sodium hydroxide to approximately the desired. Includes kit list and safety instructions. Hydrochloric acid reacts with sodium hydroxide on the. Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers.
From www.studocu.com
Analysis of an Acid by Titration with Sodium hydroxide Truc Vo 1 Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers Initially the ph is due to pure acetic acid. Includes kit list and safety instructions. As sodium hydroxide is added it reacts with the acetic acid forming its conjugate base, the salt sodium acetate. Titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. To find the concentration of sodium hydroxide by titrating hydrochloric acid with sodium hydroxide. Use. Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers.
From www.youtube.com
Tutorial Titration of Hydrochloric Acid and Sodium Hydroxide YouTube Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. As sodium hydroxide is added it reacts with the acetic acid forming its conjugate base, the salt sodium acetate. Titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. Initially the ph is due to pure acetic acid. Use this class practical to explore titration, producing the salt sodium. Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers.
From www.studocu.com
Standardization of Hydrochloric acid using Sodium Carbonate Lab Report Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. As sodium hydroxide is added it reacts with the acetic acid forming its conjugate base, the salt sodium acetate. Initially the ph is due to pure acetic acid. Usually, sodium hydroxide. Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers.
From edu.rsc.org
Titrating sodium hydroxide with hydrochloric acid Experiment RSC Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers Titration of hydrochloric acid with sodium hydroxide. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Includes kit list and safety instructions. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. Initially the ph is due to pure acetic acid. Titration of acetic acid with sodium hydroxide (analagous to figure. Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers.
From www.hotzxgirl.com
Titration Standardization Of Sodium Hydroxide Chegg Hot Sex Picture Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers Includes kit list and safety instructions. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. As sodium hydroxide is added it reacts with the acetic acid forming its conjugate base, the salt sodium acetate. Titration of hydrochloric acid with sodium hydroxide. Initially the ph is due to pure acetic acid. Usually,. Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers.
From byjus.com
Write the neutralization reaction between Hydrochloric acid HCI and Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers Includes kit list and safety instructions. Titration of hydrochloric acid with sodium hydroxide. Initially the ph is due to pure acetic acid. Titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. To find the concentration of sodium hydroxide by titrating hydrochloric acid with sodium hydroxide. Usually, sodium hydroxide solutions are prepared by diluting a 50% aqueous solution of. Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers.
From www.scribd.com
Cce48Titration of Sodium Hydroxide With Hydrochloric Acid Sodium Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers As sodium hydroxide is added it reacts with the acetic acid forming its conjugate base, the salt sodium acetate. Titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. To find the concentration of sodium hydroxide by titrating hydrochloric acid with sodium hydroxide. Usually, sodium hydroxide solutions are prepared. Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers.
From studylib.net
Titration of Hydrochloric Acid with Sodium Hydroxide Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers Titration of hydrochloric acid with sodium hydroxide. Usually, sodium hydroxide solutions are prepared by diluting a 50% aqueous solution of sodium hydroxide to approximately the desired. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Includes kit list and safety instructions. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis.. Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers.
From www.chegg.com
TitrationStandardization of Sodium Hydroxide Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers Initially the ph is due to pure acetic acid. To find the concentration of sodium hydroxide by titrating hydrochloric acid with sodium hydroxide. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. Titration of hydrochloric acid with sodium hydroxide. Includes kit list and safety instructions. As sodium hydroxide is added it reacts with the acetic acid forming its conjugate. Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers.
From teachernotes4u.com
Diagram shows the apparatus setup to study the reaction between sodium Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. As sodium hydroxide is added it reacts with the acetic acid forming its conjugate base, the salt sodium acetate. Includes kit list and safety instructions. Usually, sodium hydroxide solutions are prepared by diluting a 50% aqueous solution of sodium hydroxide to approximately the desired. To find the concentration of sodium. Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers.
From studylib.net
3998 211 course Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers Includes kit list and safety instructions. To find the concentration of sodium hydroxide by titrating hydrochloric acid with sodium hydroxide. Titration of hydrochloric acid with sodium hydroxide. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Initially the ph is due to pure acetic acid. Titration of acetic acid with sodium. Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers.
From www.tutormyself.com
(f) Acids, alkalis and titrations Archives TutorMyself Chemistry Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers Titration of hydrochloric acid with sodium hydroxide. Includes kit list and safety instructions. Titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Usually, sodium hydroxide solutions are prepared by diluting a 50% aqueous solution of sodium hydroxide to approximately the. Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers.
From www.studocu.com
Experiment 3 lab report Experiment 3 PREPARATION OF A STANDARD Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers Includes kit list and safety instructions. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. As sodium hydroxide is added it reacts with the acetic acid forming its conjugate base, the salt sodium acetate. Usually, sodium hydroxide solutions are prepared by diluting a 50% aqueous solution of sodium hydroxide to approximately the desired. Titration of hydrochloric acid with sodium. Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers.
From www.chegg.com
Solved Method Titration of sodium hydroxide and hydrochloric Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. Titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. As sodium hydroxide is added it reacts with the acetic acid forming its conjugate base, the salt sodium acetate. Initially the ph is due to pure acetic acid. To find the concentration of sodium hydroxide by titrating hydrochloric acid. Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers.
From americanessay.pages.dev
Titration Test Observation And Inference AMERICANESSAY Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers Titration of hydrochloric acid with sodium hydroxide. Titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. Usually, sodium hydroxide solutions are prepared by diluting a 50% aqueous solution of sodium hydroxide to approximately the desired. Initially the ph is due to pure acetic acid. To find the concentration. Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers.
From about.dataclassroom.com
AcidBase Titration Lab — DataClassroom Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. Titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. As sodium hydroxide is added it reacts with the acetic acid forming its conjugate base, the salt sodium acetate. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Includes kit. Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers.
From celhcddi.blob.core.windows.net
Titration Lab Math at Mike Brown blog Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers Initially the ph is due to pure acetic acid. As sodium hydroxide is added it reacts with the acetic acid forming its conjugate base, the salt sodium acetate. Includes kit list and safety instructions. Titration of hydrochloric acid with sodium hydroxide. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Usually,. Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. To find the concentration of sodium hydroxide by titrating hydrochloric acid with sodium hydroxide. Titration of hydrochloric acid with sodium hydroxide. Usually, sodium hydroxide solutions are prepared by diluting a 50% aqueous solution of sodium hydroxide to approximately the desired. Includes kit list and safety instructions. As sodium hydroxide is. Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers.
From www.chegg.com
Solved TITRATION ⋅ STANDARDIZATION OF SODIUM HYDROXIDE Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers Titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Usually, sodium hydroxide solutions are prepared by diluting a 50% aqueous solution of sodium hydroxide to approximately the desired. As sodium hydroxide is added it reacts with the acetic acid forming. Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers.
From www.chegg.com
Solved Hydrochloric acid can also be titrated with sodium Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers As sodium hydroxide is added it reacts with the acetic acid forming its conjugate base, the salt sodium acetate. Titration of hydrochloric acid with sodium hydroxide. To find the concentration of sodium hydroxide by titrating hydrochloric acid with sodium hydroxide. Initially the ph is due to pure acetic acid. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. Includes. Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers.
From www.chegg.com
TITRATION OF A HYDROCHLORIC ACID SOLUTION WITH A Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers Initially the ph is due to pure acetic acid. Usually, sodium hydroxide solutions are prepared by diluting a 50% aqueous solution of sodium hydroxide to approximately the desired. Titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. Titration of hydrochloric acid with sodium hydroxide. Includes kit list and. Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers.
From www.chegg.com
Solved Lab 12 Acid Base Titration Report Part 1 Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. Titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. To find the concentration of sodium hydroxide by titrating hydrochloric acid with sodium hydroxide. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Initially the ph is due to pure. Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers.
From www.scribd.com
Chem Lab Report Oxalic Acid Titration Sodium Hydroxide Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers Titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. Includes kit list and safety instructions. Titration of hydrochloric acid with sodium hydroxide. As sodium hydroxide is added it reacts with the acetic acid forming its conjugate base, the salt sodium acetate. To find the concentration of sodium hydroxide by titrating hydrochloric acid with sodium hydroxide. Hydrochloric acid reacts. Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers.
From www.numerade.com
SOLVED It takes 21.491 mL to reach the endpoint. For a titration of 25 Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers Titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. Initially the ph is due to pure acetic acid. Usually, sodium hydroxide solutions are prepared by diluting a 50% aqueous solution of sodium hydroxide to approximately the desired. To find the concentration of sodium hydroxide by titrating hydrochloric acid. Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers.
From studylib.net
Standardizing a Sodium Hydroxide (NaOH) Solution Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers Titration of hydrochloric acid with sodium hydroxide. Initially the ph is due to pure acetic acid. Includes kit list and safety instructions. To find the concentration of sodium hydroxide by titrating hydrochloric acid with sodium hydroxide. As sodium hydroxide is added it reacts with the acetic acid forming its conjugate base, the salt sodium acetate. Titration of acetic acid with. Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers.
From www.thinkswap.com
Titration of Sodium Hydroxide with Hydrochloric acid FSC107 General Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. As sodium hydroxide is added it reacts with the acetic acid forming its conjugate base, the salt sodium acetate. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Titration of hydrochloric acid with sodium hydroxide. Initially the ph is due to. Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers.
From www.numerade.com
SOLVEDmEztion CUNVELAFnment; 1) Predict the titration curve for the Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers Titration of hydrochloric acid with sodium hydroxide. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. Titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. Includes kit list and safety instructions. As sodium hydroxide is added it reacts with the acetic acid forming its conjugate base, the salt sodium acetate. To find the concentration of sodium hydroxide. Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers.
From www.chegg.com
Solved TITRATION STANDARDIZATION OF SODIUM HYDROXIDE Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers As sodium hydroxide is added it reacts with the acetic acid forming its conjugate base, the salt sodium acetate. Titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. To find the concentration of sodium hydroxide by titrating hydrochloric acid with sodium hydroxide. Usually, sodium hydroxide solutions are prepared. Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers.
From www.numerade.com
SOLVED The following data is from a conductometric titration of 10.00 Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers Initially the ph is due to pure acetic acid. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. Includes kit list and safety instructions. As sodium hydroxide is added it reacts with the acetic acid forming its conjugate base, the salt. Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers.
From www.studypool.com
SOLUTION CHM 113 Experiment 8 Acid Base Titration Lab Report Studypool Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers Includes kit list and safety instructions. To find the concentration of sodium hydroxide by titrating hydrochloric acid with sodium hydroxide. Usually, sodium hydroxide solutions are prepared by diluting a 50% aqueous solution of sodium hydroxide to approximately the desired. Initially the ph is due to pure acetic acid. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. Titration of. Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers.
From webapi.bu.edu
Standardizing a solution of sodium hydroxide lab report answers. Lab Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers Titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Usually, sodium hydroxide solutions are prepared by diluting a 50% aqueous solution of sodium hydroxide to approximately the desired. To find the concentration of sodium hydroxide by titrating hydrochloric acid with. Titration Of Hydrochloric Acid With Sodium Hydroxide Lab Report Answers.