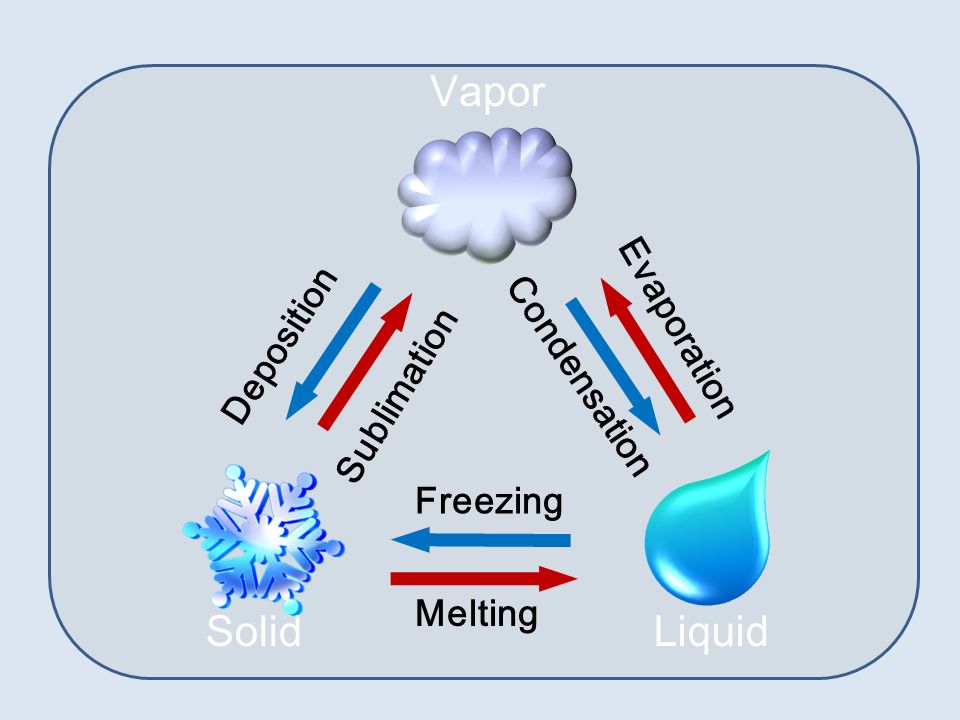
Example Of Condensation And Sublimation . sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. condensation occurs when a gas/vapor loses energy or gains pressure to move to a liquid state. examples and applications of sublimation. for example, if you view the sublimation of dry ice into carbon dioxide gas, the white vapor that is observed is mostly water that is condensing. define melting, freezing, vaporization, condensation, sublimation, and deposition. sublimation is the change of state from a solid to a gas, without passing through the liquid state. Sublimation is the transition of a substance directly from the solid phase to the gas phase without passing through. condensation involves the transition from a gaseous state to a liquid state, bypassing the solid phase. sublimation is defined as a process in which solid converts into gas directly without converting into liquid.
from app.emaze.com
sublimation is defined as a process in which solid converts into gas directly without converting into liquid. examples and applications of sublimation. condensation occurs when a gas/vapor loses energy or gains pressure to move to a liquid state. sublimation is the change of state from a solid to a gas, without passing through the liquid state. Sublimation is the transition of a substance directly from the solid phase to the gas phase without passing through. sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. condensation involves the transition from a gaseous state to a liquid state, bypassing the solid phase. for example, if you view the sublimation of dry ice into carbon dioxide gas, the white vapor that is observed is mostly water that is condensing. define melting, freezing, vaporization, condensation, sublimation, and deposition.
on emaze
Example Of Condensation And Sublimation condensation involves the transition from a gaseous state to a liquid state, bypassing the solid phase. define melting, freezing, vaporization, condensation, sublimation, and deposition. sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. sublimation is defined as a process in which solid converts into gas directly without converting into liquid. condensation occurs when a gas/vapor loses energy or gains pressure to move to a liquid state. examples and applications of sublimation. sublimation is the change of state from a solid to a gas, without passing through the liquid state. Sublimation is the transition of a substance directly from the solid phase to the gas phase without passing through. for example, if you view the sublimation of dry ice into carbon dioxide gas, the white vapor that is observed is mostly water that is condensing. condensation involves the transition from a gaseous state to a liquid state, bypassing the solid phase.
From www.dryicecorp.com
What Is Sublimation? Dry Ice Corp Example Of Condensation And Sublimation sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. Sublimation is the transition of a substance directly from the solid phase to the gas phase without passing through. examples and applications of sublimation. define melting, freezing, vaporization, condensation, sublimation, and deposition. sublimation is defined as a process. Example Of Condensation And Sublimation.
From byjus.com
Give one simple activity/experiment to show that ammonium chloride Example Of Condensation And Sublimation Sublimation is the transition of a substance directly from the solid phase to the gas phase without passing through. sublimation is defined as a process in which solid converts into gas directly without converting into liquid. examples and applications of sublimation. define melting, freezing, vaporization, condensation, sublimation, and deposition. condensation occurs when a gas/vapor loses energy. Example Of Condensation And Sublimation.
From www.youtube.com
Condensation, Deposition, and Sublimation YouTube Example Of Condensation And Sublimation sublimation is the change of state from a solid to a gas, without passing through the liquid state. condensation involves the transition from a gaseous state to a liquid state, bypassing the solid phase. for example, if you view the sublimation of dry ice into carbon dioxide gas, the white vapor that is observed is mostly water. Example Of Condensation And Sublimation.
From www.youtube.com
To Observe the Process of Sublimation Class5 YouTube Example Of Condensation And Sublimation condensation involves the transition from a gaseous state to a liquid state, bypassing the solid phase. sublimation is the change of state from a solid to a gas, without passing through the liquid state. define melting, freezing, vaporization, condensation, sublimation, and deposition. sublimation is defined as a process in which solid converts into gas directly without. Example Of Condensation And Sublimation.
From www.slideserve.com
PPT Changes in State of Matter PowerPoint Presentation, free download Example Of Condensation And Sublimation define melting, freezing, vaporization, condensation, sublimation, and deposition. condensation involves the transition from a gaseous state to a liquid state, bypassing the solid phase. examples and applications of sublimation. for example, if you view the sublimation of dry ice into carbon dioxide gas, the white vapor that is observed is mostly water that is condensing. . Example Of Condensation And Sublimation.
From www.thoughtco.com
Sublimation Definition (Phase Transition in Chemistry) Example Of Condensation And Sublimation condensation occurs when a gas/vapor loses energy or gains pressure to move to a liquid state. sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. sublimation is the change of state from a solid to a gas, without passing through the liquid state. define melting, freezing, vaporization,. Example Of Condensation And Sublimation.
From www.slideserve.com
PPT States of Matter Phase Change PowerPoint Presentation ID1115834 Example Of Condensation And Sublimation Sublimation is the transition of a substance directly from the solid phase to the gas phase without passing through. sublimation is the change of state from a solid to a gas, without passing through the liquid state. sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. sublimation is. Example Of Condensation And Sublimation.
From www.teachoo.com
What is Sublimation? 5+ Examples with Diagram Teachoo Example Of Condensation And Sublimation condensation involves the transition from a gaseous state to a liquid state, bypassing the solid phase. condensation occurs when a gas/vapor loses energy or gains pressure to move to a liquid state. Sublimation is the transition of a substance directly from the solid phase to the gas phase without passing through. sublimation is the change of state. Example Of Condensation And Sublimation.
From www.slideshare.net
(Science) Matter Example Of Condensation And Sublimation condensation occurs when a gas/vapor loses energy or gains pressure to move to a liquid state. Sublimation is the transition of a substance directly from the solid phase to the gas phase without passing through. condensation involves the transition from a gaseous state to a liquid state, bypassing the solid phase. sublimation, in physics, conversion of a. Example Of Condensation And Sublimation.
From advergize.com
5+ Sublimation Examples in Everyday Life Example Of Condensation And Sublimation condensation occurs when a gas/vapor loses energy or gains pressure to move to a liquid state. sublimation is defined as a process in which solid converts into gas directly without converting into liquid. examples and applications of sublimation. condensation involves the transition from a gaseous state to a liquid state, bypassing the solid phase. for. Example Of Condensation And Sublimation.
From studiousguy.com
7 Sublimation Examples in Daily Life StudiousGuy Example Of Condensation And Sublimation define melting, freezing, vaporization, condensation, sublimation, and deposition. condensation occurs when a gas/vapor loses energy or gains pressure to move to a liquid state. examples and applications of sublimation. Sublimation is the transition of a substance directly from the solid phase to the gas phase without passing through. sublimation, in physics, conversion of a substance from. Example Of Condensation And Sublimation.
From kaia-has-orr.blogspot.com
Explain the Difference Between Evaporation and Sublimation KaiahasOrr Example Of Condensation And Sublimation for example, if you view the sublimation of dry ice into carbon dioxide gas, the white vapor that is observed is mostly water that is condensing. condensation occurs when a gas/vapor loses energy or gains pressure to move to a liquid state. sublimation is defined as a process in which solid converts into gas directly without converting. Example Of Condensation And Sublimation.
From www.britannica.com
Sublimation Definition, Examples, & Facts Britannica Example Of Condensation And Sublimation Sublimation is the transition of a substance directly from the solid phase to the gas phase without passing through. sublimation is defined as a process in which solid converts into gas directly without converting into liquid. condensation occurs when a gas/vapor loses energy or gains pressure to move to a liquid state. define melting, freezing, vaporization, condensation,. Example Of Condensation And Sublimation.
From stock.adobe.com
Vector diagram with changing states of matter, three states of matter Example Of Condensation And Sublimation define melting, freezing, vaporization, condensation, sublimation, and deposition. condensation involves the transition from a gaseous state to a liquid state, bypassing the solid phase. examples and applications of sublimation. sublimation is defined as a process in which solid converts into gas directly without converting into liquid. Sublimation is the transition of a substance directly from the. Example Of Condensation And Sublimation.
From www.youtube.com
Change of State Evaporation Condensation Melting Freezing Example Of Condensation And Sublimation examples and applications of sublimation. sublimation is defined as a process in which solid converts into gas directly without converting into liquid. condensation involves the transition from a gaseous state to a liquid state, bypassing the solid phase. sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid.. Example Of Condensation And Sublimation.
From www.science-sparks.com
What is sublimation? Example Of Condensation And Sublimation Sublimation is the transition of a substance directly from the solid phase to the gas phase without passing through. define melting, freezing, vaporization, condensation, sublimation, and deposition. sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. sublimation is the change of state from a solid to a gas,. Example Of Condensation And Sublimation.
From www.pinterest.co.uk
Pin on Diagrams and Infographics Example Of Condensation And Sublimation condensation occurs when a gas/vapor loses energy or gains pressure to move to a liquid state. for example, if you view the sublimation of dry ice into carbon dioxide gas, the white vapor that is observed is mostly water that is condensing. condensation involves the transition from a gaseous state to a liquid state, bypassing the solid. Example Of Condensation And Sublimation.
From www.vrogue.co
Bbc Science Worksheet Evaporation And Condensation Ev vrogue.co Example Of Condensation And Sublimation define melting, freezing, vaporization, condensation, sublimation, and deposition. sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. sublimation is the change of state from a solid to a gas, without passing through the liquid state. condensation occurs when a gas/vapor loses energy or gains pressure to move. Example Of Condensation And Sublimation.
From www.vrogue.co
What Is Sublimation Its Process Examples And Uses vrogue.co Example Of Condensation And Sublimation condensation occurs when a gas/vapor loses energy or gains pressure to move to a liquid state. condensation involves the transition from a gaseous state to a liquid state, bypassing the solid phase. sublimation is the change of state from a solid to a gas, without passing through the liquid state. sublimation, in physics, conversion of a. Example Of Condensation And Sublimation.
From www.expii.com
Sublimation — Definition & Overview Expii Example Of Condensation And Sublimation for example, if you view the sublimation of dry ice into carbon dioxide gas, the white vapor that is observed is mostly water that is condensing. Sublimation is the transition of a substance directly from the solid phase to the gas phase without passing through. sublimation is defined as a process in which solid converts into gas directly. Example Of Condensation And Sublimation.
From www.bajeczneobrazy.pl
Physical states of matter.Solid, liquid and gas.Melting, freezing Example Of Condensation And Sublimation condensation occurs when a gas/vapor loses energy or gains pressure to move to a liquid state. sublimation is defined as a process in which solid converts into gas directly without converting into liquid. sublimation is the change of state from a solid to a gas, without passing through the liquid state. sublimation, in physics, conversion of. Example Of Condensation And Sublimation.
From facts.net
20 Astounding Facts About Sublimation Example Of Condensation And Sublimation examples and applications of sublimation. sublimation is defined as a process in which solid converts into gas directly without converting into liquid. define melting, freezing, vaporization, condensation, sublimation, and deposition. sublimation is the change of state from a solid to a gas, without passing through the liquid state. Sublimation is the transition of a substance directly. Example Of Condensation And Sublimation.
From www.tec-science.com
Specific latent heat of condensation tecscience Example Of Condensation And Sublimation sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. define melting, freezing, vaporization, condensation, sublimation, and deposition. sublimation is the change of state from a solid to a gas, without passing through the liquid state. condensation occurs when a gas/vapor loses energy or gains pressure to move. Example Of Condensation And Sublimation.
From www.studyread.com
What is sublimation Its process, examples and uses Example Of Condensation And Sublimation Sublimation is the transition of a substance directly from the solid phase to the gas phase without passing through. define melting, freezing, vaporization, condensation, sublimation, and deposition. condensation occurs when a gas/vapor loses energy or gains pressure to move to a liquid state. condensation involves the transition from a gaseous state to a liquid state, bypassing the. Example Of Condensation And Sublimation.
From www.youtube.com
Phase Transitions Freezing, Melting, Condensation, Evaporation, and Example Of Condensation And Sublimation sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. sublimation is the change of state from a solid to a gas, without passing through the liquid state. condensation involves the transition from a gaseous state to a liquid state, bypassing the solid phase. define melting, freezing, vaporization,. Example Of Condensation And Sublimation.
From circuitgonelladrianxm.z22.web.core.windows.net
Show The Phase Change Diagram Example Of Condensation And Sublimation condensation involves the transition from a gaseous state to a liquid state, bypassing the solid phase. sublimation is the change of state from a solid to a gas, without passing through the liquid state. sublimation is defined as a process in which solid converts into gas directly without converting into liquid. examples and applications of sublimation.. Example Of Condensation And Sublimation.
From www.youtube.com
Changing states of matter 🔁 Melting, Freezing, Evaporation Example Of Condensation And Sublimation condensation occurs when a gas/vapor loses energy or gains pressure to move to a liquid state. sublimation is the change of state from a solid to a gas, without passing through the liquid state. condensation involves the transition from a gaseous state to a liquid state, bypassing the solid phase. sublimation, in physics, conversion of a. Example Of Condensation And Sublimation.
From sciencing.com
The Difference Between Deposition & Sublimation Sciencing Example Of Condensation And Sublimation sublimation is defined as a process in which solid converts into gas directly without converting into liquid. condensation occurs when a gas/vapor loses energy or gains pressure to move to a liquid state. sublimation is the change of state from a solid to a gas, without passing through the liquid state. for example, if you view. Example Of Condensation And Sublimation.
From circuitgonelladrianxm.z22.web.core.windows.net
Phase Diagram Phase Changes Example Of Condensation And Sublimation define melting, freezing, vaporization, condensation, sublimation, and deposition. condensation involves the transition from a gaseous state to a liquid state, bypassing the solid phase. sublimation is the change of state from a solid to a gas, without passing through the liquid state. sublimation, in physics, conversion of a substance from the solid to the gaseous state. Example Of Condensation And Sublimation.
From apollo.lsc.vsc.edu
Latent Heats sublimation and deposition Example Of Condensation And Sublimation examples and applications of sublimation. sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. condensation involves the transition from a gaseous state to a liquid state, bypassing the solid phase. Sublimation is the transition of a substance directly from the solid phase to the gas phase without passing. Example Of Condensation And Sublimation.
From mysixthclass.blogspot.com
Sublimation My Sixth Class Example Of Condensation And Sublimation Sublimation is the transition of a substance directly from the solid phase to the gas phase without passing through. examples and applications of sublimation. sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. condensation involves the transition from a gaseous state to a liquid state, bypassing the solid. Example Of Condensation And Sublimation.
From app.emaze.com
on emaze Example Of Condensation And Sublimation define melting, freezing, vaporization, condensation, sublimation, and deposition. sublimation is the change of state from a solid to a gas, without passing through the liquid state. sublimation is defined as a process in which solid converts into gas directly without converting into liquid. sublimation, in physics, conversion of a substance from the solid to the gaseous. Example Of Condensation And Sublimation.
From brainly.ph
Draw an example of each phase change that you can observe at home Example Of Condensation And Sublimation condensation occurs when a gas/vapor loses energy or gains pressure to move to a liquid state. sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. Sublimation is the transition of a substance directly from the solid phase to the gas phase without passing through. sublimation is the change. Example Of Condensation And Sublimation.
From atocwatervapor.blogspot.com
Water Phase Change Sublimation Example Of Condensation And Sublimation sublimation, in physics, conversion of a substance from the solid to the gaseous state without its becoming liquid. sublimation is the change of state from a solid to a gas, without passing through the liquid state. sublimation is defined as a process in which solid converts into gas directly without converting into liquid. Sublimation is the transition. Example Of Condensation And Sublimation.
From www.geeksforgeeks.org
What is Vaporization? Example Of Condensation And Sublimation sublimation is defined as a process in which solid converts into gas directly without converting into liquid. condensation occurs when a gas/vapor loses energy or gains pressure to move to a liquid state. sublimation is the change of state from a solid to a gas, without passing through the liquid state. Sublimation is the transition of a. Example Of Condensation And Sublimation.