Titration Curve Ka . sketch titration curves for the following two systems: how to find the acid dissociation constant, ka, for an acid using a titration curve. in this video, i will teach you how to calculate the pka and the ka simply. H+(aq) ion concentration and half equivalence point. in this experiment you will learn to calibrate and use a ph probe, and then construct a titration curve (graph) in order to determine the molarity and ka (acid. It determines the dissociation of acid in an. ka or dissociation constant is a standard used to measure the acidic strength. (a) the titration of 50.0 ml of 0.050 m h 2 a, a diprotic weak acid with a pk a1 of 3 and a pk a2 of 7;.
from chem.libretexts.org
in this experiment you will learn to calibrate and use a ph probe, and then construct a titration curve (graph) in order to determine the molarity and ka (acid. in this video, i will teach you how to calculate the pka and the ka simply. It determines the dissociation of acid in an. how to find the acid dissociation constant, ka, for an acid using a titration curve. sketch titration curves for the following two systems: H+(aq) ion concentration and half equivalence point. (a) the titration of 50.0 ml of 0.050 m h 2 a, a diprotic weak acid with a pk a1 of 3 and a pk a2 of 7;. ka or dissociation constant is a standard used to measure the acidic strength.
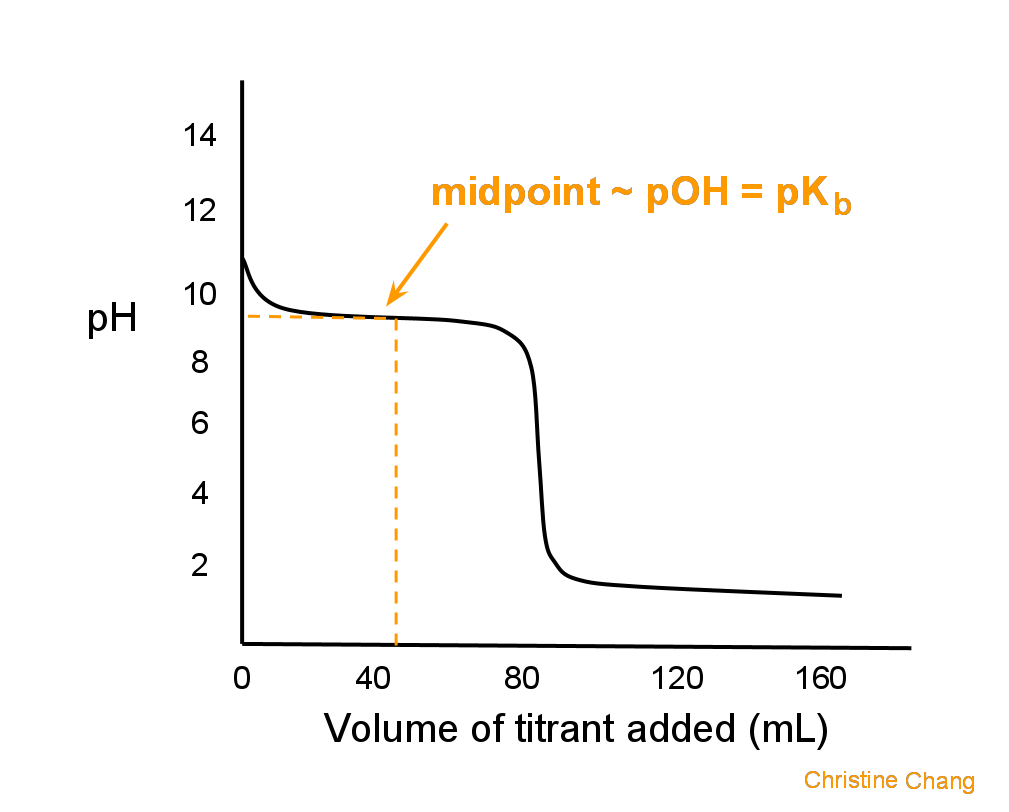
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts
Titration Curve Ka in this experiment you will learn to calibrate and use a ph probe, and then construct a titration curve (graph) in order to determine the molarity and ka (acid. ka or dissociation constant is a standard used to measure the acidic strength. how to find the acid dissociation constant, ka, for an acid using a titration curve. H+(aq) ion concentration and half equivalence point. in this video, i will teach you how to calculate the pka and the ka simply. (a) the titration of 50.0 ml of 0.050 m h 2 a, a diprotic weak acid with a pk a1 of 3 and a pk a2 of 7;. in this experiment you will learn to calibrate and use a ph probe, and then construct a titration curve (graph) in order to determine the molarity and ka (acid. sketch titration curves for the following two systems: It determines the dissociation of acid in an.
From mavink.com
Acid Base Titration Curve Titration Curve Ka how to find the acid dissociation constant, ka, for an acid using a titration curve. H+(aq) ion concentration and half equivalence point. It determines the dissociation of acid in an. (a) the titration of 50.0 ml of 0.050 m h 2 a, a diprotic weak acid with a pk a1 of 3 and a pk a2 of 7;. . Titration Curve Ka.
From courses.lumenlearning.com
14.8 AcidBase Titrations General College Chemistry II Titration Curve Ka It determines the dissociation of acid in an. how to find the acid dissociation constant, ka, for an acid using a titration curve. in this experiment you will learn to calibrate and use a ph probe, and then construct a titration curve (graph) in order to determine the molarity and ka (acid. sketch titration curves for the. Titration Curve Ka.
From www.youtube.com
Titration Curves, Equivalence Point YouTube Titration Curve Ka It determines the dissociation of acid in an. ka or dissociation constant is a standard used to measure the acidic strength. (a) the titration of 50.0 ml of 0.050 m h 2 a, a diprotic weak acid with a pk a1 of 3 and a pk a2 of 7;. sketch titration curves for the following two systems: . Titration Curve Ka.
From www.writework.com
Titration of amino acids WriteWork Titration Curve Ka how to find the acid dissociation constant, ka, for an acid using a titration curve. ka or dissociation constant is a standard used to measure the acidic strength. (a) the titration of 50.0 ml of 0.050 m h 2 a, a diprotic weak acid with a pk a1 of 3 and a pk a2 of 7;. in. Titration Curve Ka.
From exogrnoza.blob.core.windows.net
Titration Curve Acetic Acid And Naoh at Isabel Keith blog Titration Curve Ka It determines the dissociation of acid in an. in this video, i will teach you how to calculate the pka and the ka simply. H+(aq) ion concentration and half equivalence point. ka or dissociation constant is a standard used to measure the acidic strength. how to find the acid dissociation constant, ka, for an acid using a. Titration Curve Ka.
From www.grace.umd.edu
Simulation of Monoprotic Titration Curve Titration Curve Ka H+(aq) ion concentration and half equivalence point. sketch titration curves for the following two systems: ka or dissociation constant is a standard used to measure the acidic strength. how to find the acid dissociation constant, ka, for an acid using a titration curve. (a) the titration of 50.0 ml of 0.050 m h 2 a, a diprotic. Titration Curve Ka.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Titration Curve Ka how to find the acid dissociation constant, ka, for an acid using a titration curve. H+(aq) ion concentration and half equivalence point. (a) the titration of 50.0 ml of 0.050 m h 2 a, a diprotic weak acid with a pk a1 of 3 and a pk a2 of 7;. ka or dissociation constant is a standard used. Titration Curve Ka.
From dxobegonq.blob.core.windows.net
Titration Curve Labels at Ok Moore blog Titration Curve Ka sketch titration curves for the following two systems: H+(aq) ion concentration and half equivalence point. It determines the dissociation of acid in an. in this experiment you will learn to calibrate and use a ph probe, and then construct a titration curve (graph) in order to determine the molarity and ka (acid. in this video, i will. Titration Curve Ka.
From 88guru.com
Acid Base Titration What is a Titration Curve? 88guru Titration Curve Ka in this experiment you will learn to calibrate and use a ph probe, and then construct a titration curve (graph) in order to determine the molarity and ka (acid. It determines the dissociation of acid in an. sketch titration curves for the following two systems: ka or dissociation constant is a standard used to measure the acidic. Titration Curve Ka.
From exoapenql.blob.core.windows.net
Titration Curve Ka Value at Clarence Ellsworth blog Titration Curve Ka ka or dissociation constant is a standard used to measure the acidic strength. in this video, i will teach you how to calculate the pka and the ka simply. It determines the dissociation of acid in an. (a) the titration of 50.0 ml of 0.050 m h 2 a, a diprotic weak acid with a pk a1 of. Titration Curve Ka.
From chem.libretexts.org
3.7 AcidBase Titrations Chemistry LibreTexts Titration Curve Ka in this video, i will teach you how to calculate the pka and the ka simply. ka or dissociation constant is a standard used to measure the acidic strength. sketch titration curves for the following two systems: how to find the acid dissociation constant, ka, for an acid using a titration curve. in this experiment. Titration Curve Ka.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Curve Ka how to find the acid dissociation constant, ka, for an acid using a titration curve. (a) the titration of 50.0 ml of 0.050 m h 2 a, a diprotic weak acid with a pk a1 of 3 and a pk a2 of 7;. in this video, i will teach you how to calculate the pka and the ka. Titration Curve Ka.
From oneclass.com
OneClass 2. (a) From the titration curve given below, calculate the Ka Titration Curve Ka in this video, i will teach you how to calculate the pka and the ka simply. how to find the acid dissociation constant, ka, for an acid using a titration curve. ka or dissociation constant is a standard used to measure the acidic strength. It determines the dissociation of acid in an. in this experiment you. Titration Curve Ka.
From morioh.com
Acid Base Titration Curves PH Calculations Titration Curve Ka sketch titration curves for the following two systems: (a) the titration of 50.0 ml of 0.050 m h 2 a, a diprotic weak acid with a pk a1 of 3 and a pk a2 of 7;. It determines the dissociation of acid in an. how to find the acid dissociation constant, ka, for an acid using a titration. Titration Curve Ka.
From hxeqgqbgy.blob.core.windows.net
Titration Particle Diagram at Maxine Hatch blog Titration Curve Ka in this experiment you will learn to calibrate and use a ph probe, and then construct a titration curve (graph) in order to determine the molarity and ka (acid. how to find the acid dissociation constant, ka, for an acid using a titration curve. ka or dissociation constant is a standard used to measure the acidic strength.. Titration Curve Ka.
From exosxgjvz.blob.core.windows.net
Weak Acid Titration Curve Buffer Region at Paula Rivera blog Titration Curve Ka H+(aq) ion concentration and half equivalence point. It determines the dissociation of acid in an. in this experiment you will learn to calibrate and use a ph probe, and then construct a titration curve (graph) in order to determine the molarity and ka (acid. sketch titration curves for the following two systems: ka or dissociation constant is. Titration Curve Ka.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Titration Curve Ka (a) the titration of 50.0 ml of 0.050 m h 2 a, a diprotic weak acid with a pk a1 of 3 and a pk a2 of 7;. H+(aq) ion concentration and half equivalence point. how to find the acid dissociation constant, ka, for an acid using a titration curve. in this experiment you will learn to calibrate. Titration Curve Ka.
From www.youtube.com
titration curve pH calculations YouTube Titration Curve Ka (a) the titration of 50.0 ml of 0.050 m h 2 a, a diprotic weak acid with a pk a1 of 3 and a pk a2 of 7;. how to find the acid dissociation constant, ka, for an acid using a titration curve. ka or dissociation constant is a standard used to measure the acidic strength. It determines. Titration Curve Ka.
From hxetlxxnv.blob.core.windows.net
Titration Curve Important Points at Garth Burgos blog Titration Curve Ka how to find the acid dissociation constant, ka, for an acid using a titration curve. H+(aq) ion concentration and half equivalence point. It determines the dissociation of acid in an. in this video, i will teach you how to calculate the pka and the ka simply. ka or dissociation constant is a standard used to measure the. Titration Curve Ka.
From chem.libretexts.org
17.4 Neutralization Reactions and Titration Curves Chemistry LibreTexts Titration Curve Ka It determines the dissociation of acid in an. H+(aq) ion concentration and half equivalence point. in this experiment you will learn to calibrate and use a ph probe, and then construct a titration curve (graph) in order to determine the molarity and ka (acid. sketch titration curves for the following two systems: (a) the titration of 50.0 ml. Titration Curve Ka.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax Titration Curve Ka in this experiment you will learn to calibrate and use a ph probe, and then construct a titration curve (graph) in order to determine the molarity and ka (acid. (a) the titration of 50.0 ml of 0.050 m h 2 a, a diprotic weak acid with a pk a1 of 3 and a pk a2 of 7;. how. Titration Curve Ka.
From saylordotorg.github.io
AcidBase Titrations Titration Curve Ka sketch titration curves for the following two systems: in this experiment you will learn to calibrate and use a ph probe, and then construct a titration curve (graph) in order to determine the molarity and ka (acid. ka or dissociation constant is a standard used to measure the acidic strength. how to find the acid dissociation. Titration Curve Ka.
From www.chegg.com
Solved The graph shows the titration curves of a strong acid Titration Curve Ka in this video, i will teach you how to calculate the pka and the ka simply. in this experiment you will learn to calibrate and use a ph probe, and then construct a titration curve (graph) in order to determine the molarity and ka (acid. It determines the dissociation of acid in an. how to find the. Titration Curve Ka.
From hxeqgqbgy.blob.core.windows.net
Titration Particle Diagram at Maxine Hatch blog Titration Curve Ka ka or dissociation constant is a standard used to measure the acidic strength. in this experiment you will learn to calibrate and use a ph probe, and then construct a titration curve (graph) in order to determine the molarity and ka (acid. how to find the acid dissociation constant, ka, for an acid using a titration curve.. Titration Curve Ka.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration Curve Ka It determines the dissociation of acid in an. H+(aq) ion concentration and half equivalence point. sketch titration curves for the following two systems: in this experiment you will learn to calibrate and use a ph probe, and then construct a titration curve (graph) in order to determine the molarity and ka (acid. how to find the acid. Titration Curve Ka.
From hxeqgqbgy.blob.core.windows.net
Titration Particle Diagram at Maxine Hatch blog Titration Curve Ka how to find the acid dissociation constant, ka, for an acid using a titration curve. It determines the dissociation of acid in an. H+(aq) ion concentration and half equivalence point. in this video, i will teach you how to calculate the pka and the ka simply. sketch titration curves for the following two systems: (a) the titration. Titration Curve Ka.