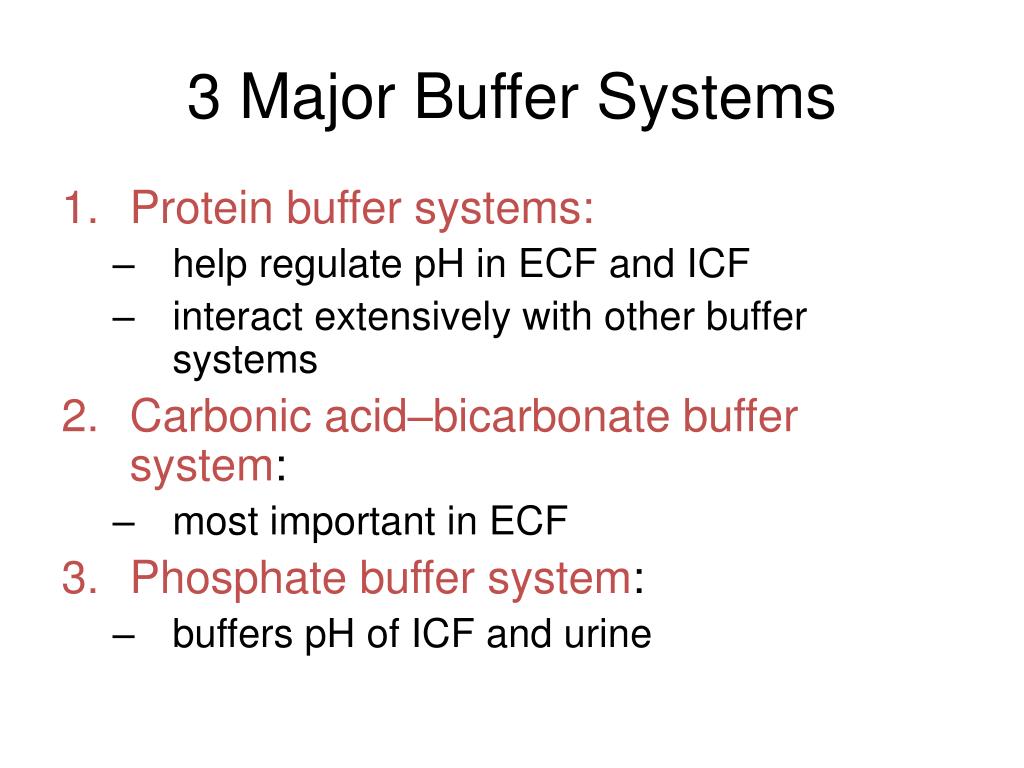
What Are The 3 Buffer Systems . A buffer is a solution that can withstand changes in ph. The bicarbonate buffer system can act within seconds to minutes to counteract changes in ph, while the lungs take minutes, and the kidneys take hours to days. It is caused by the addition of acidic or basic components. The bicarbonate buffer system manages acid/base imbalances and effectively manages the release of excess carbon dioxide as a bi. A solution used to stabilize the ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Blood ph is regulated by multiple homeostatic mechanisms, including chemical buffers, respiration, and the kidneys.
from www.slideserve.com
Blood ph is regulated by multiple homeostatic mechanisms, including chemical buffers, respiration, and the kidneys. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The bicarbonate buffer system manages acid/base imbalances and effectively manages the release of excess carbon dioxide as a bi. A buffer is a solution that can withstand changes in ph. The bicarbonate buffer system can act within seconds to minutes to counteract changes in ph, while the lungs take minutes, and the kidneys take hours to days. It is caused by the addition of acidic or basic components. A solution used to stabilize the ph.
PPT Chapter 26 Balance PowerPoint Presentation, free download ID
What Are The 3 Buffer Systems Blood ph is regulated by multiple homeostatic mechanisms, including chemical buffers, respiration, and the kidneys. It is caused by the addition of acidic or basic components. Blood ph is regulated by multiple homeostatic mechanisms, including chemical buffers, respiration, and the kidneys. The bicarbonate buffer system manages acid/base imbalances and effectively manages the release of excess carbon dioxide as a bi. The bicarbonate buffer system can act within seconds to minutes to counteract changes in ph, while the lungs take minutes, and the kidneys take hours to days. A solution used to stabilize the ph. A buffer is a solution that can withstand changes in ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers.
From www.slideserve.com
PPT Acid, Base, Electrolytes PowerPoint Presentation, free download What Are The 3 Buffer Systems A buffer is a solution that can withstand changes in ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. It is caused by the addition of acidic or basic components. A solution used to stabilize the ph. The bicarbonate buffer system manages acid/base imbalances and effectively manages the release of excess. What Are The 3 Buffer Systems.
From www.slideserve.com
PPT AcidBase Buffers PowerPoint Presentation ID496487 What Are The 3 Buffer Systems Blood ph is regulated by multiple homeostatic mechanisms, including chemical buffers, respiration, and the kidneys. The bicarbonate buffer system manages acid/base imbalances and effectively manages the release of excess carbon dioxide as a bi. It is caused by the addition of acidic or basic components. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic. What Are The 3 Buffer Systems.
From www.slideshare.net
Buffer system What Are The 3 Buffer Systems Blood ph is regulated by multiple homeostatic mechanisms, including chemical buffers, respiration, and the kidneys. The bicarbonate buffer system manages acid/base imbalances and effectively manages the release of excess carbon dioxide as a bi. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. A buffer is a solution that can withstand changes. What Are The 3 Buffer Systems.
From quizlet.com
Bicarbonate Buffer system Diagram Quizlet What Are The 3 Buffer Systems The bicarbonate buffer system manages acid/base imbalances and effectively manages the release of excess carbon dioxide as a bi. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. A solution used to stabilize the ph. The bicarbonate buffer system can act within seconds to minutes to counteract changes in ph, while the. What Are The 3 Buffer Systems.
From www.slideserve.com
PPT Chapter 13 AcidBase Balance PowerPoint Presentation, free What Are The 3 Buffer Systems A solution used to stabilize the ph. It is caused by the addition of acidic or basic components. Blood ph is regulated by multiple homeostatic mechanisms, including chemical buffers, respiration, and the kidneys. The bicarbonate buffer system can act within seconds to minutes to counteract changes in ph, while the lungs take minutes, and the kidneys take hours to days.. What Are The 3 Buffer Systems.
From www.slideserve.com
PPT 1. Body Fluid Compartments PowerPoint Presentation, free download What Are The 3 Buffer Systems The bicarbonate buffer system can act within seconds to minutes to counteract changes in ph, while the lungs take minutes, and the kidneys take hours to days. Blood ph is regulated by multiple homeostatic mechanisms, including chemical buffers, respiration, and the kidneys. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The. What Are The 3 Buffer Systems.
From www.slideserve.com
PPT Chapter 27 Fluid, Electrolyte and Acidbase Balance PowerPoint What Are The 3 Buffer Systems Blood ph is regulated by multiple homeostatic mechanisms, including chemical buffers, respiration, and the kidneys. A solution used to stabilize the ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The bicarbonate buffer system can act within seconds to minutes to counteract changes in ph, while the lungs take minutes, and. What Are The 3 Buffer Systems.
From www.youtube.com
Buffer Capacity 03 Buffers in pharmaceutical and biological systems What Are The 3 Buffer Systems A solution used to stabilize the ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. It is caused by the addition of acidic or basic components. The bicarbonate buffer system can act within seconds to minutes to counteract changes in ph, while the lungs take minutes, and the kidneys take hours. What Are The 3 Buffer Systems.
From www.myxxgirl.com
Buffer System Classification Example And Mechanism Of Action My XXX What Are The 3 Buffer Systems Blood ph is regulated by multiple homeostatic mechanisms, including chemical buffers, respiration, and the kidneys. It is caused by the addition of acidic or basic components. A buffer is a solution that can withstand changes in ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The bicarbonate buffer system manages acid/base. What Are The 3 Buffer Systems.
From www.slideserve.com
PPT ACE THE ACID BASE DISORDERS PowerPoint Presentation, free What Are The 3 Buffer Systems The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. It is caused by the addition of acidic or basic components. The bicarbonate buffer system can act within seconds to minutes to counteract changes in ph, while the lungs take minutes, and the kidneys take hours to days. A solution used to stabilize. What Are The 3 Buffer Systems.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint What Are The 3 Buffer Systems The bicarbonate buffer system can act within seconds to minutes to counteract changes in ph, while the lungs take minutes, and the kidneys take hours to days. A solution used to stabilize the ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. A buffer is a solution that can withstand changes. What Are The 3 Buffer Systems.
From study.com
Buffer System in Chemistry Definition & Overview Video & Lesson What Are The 3 Buffer Systems The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The bicarbonate buffer system manages acid/base imbalances and effectively manages the release of excess carbon dioxide as a bi. A solution used to stabilize the ph. It is caused by the addition of acidic or basic components. The bicarbonate buffer system can act. What Are The 3 Buffer Systems.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID What Are The 3 Buffer Systems The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The bicarbonate buffer system manages acid/base imbalances and effectively manages the release of excess carbon dioxide as a bi. A solution used to stabilize the ph. The bicarbonate buffer system can act within seconds to minutes to counteract changes in ph, while the. What Are The 3 Buffer Systems.
From www.slideserve.com
PPT Buffer Systems of the Body PowerPoint Presentation, free download What Are The 3 Buffer Systems The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. A solution used to stabilize the ph. The bicarbonate buffer system can act within seconds to minutes to counteract changes in ph, while the lungs take minutes, and the kidneys take hours to days. The bicarbonate buffer system manages acid/base imbalances and effectively. What Are The 3 Buffer Systems.
From www.slideserve.com
PPT Water & Buffers PowerPoint Presentation, free download ID3886735 What Are The 3 Buffer Systems The bicarbonate buffer system can act within seconds to minutes to counteract changes in ph, while the lungs take minutes, and the kidneys take hours to days. A solution used to stabilize the ph. The bicarbonate buffer system manages acid/base imbalances and effectively manages the release of excess carbon dioxide as a bi. A buffer is a solution that can. What Are The 3 Buffer Systems.
From en.ppt-online.org
Solutions. Acidbase equilibrium in biological systems online What Are The 3 Buffer Systems A buffer is a solution that can withstand changes in ph. The bicarbonate buffer system can act within seconds to minutes to counteract changes in ph, while the lungs take minutes, and the kidneys take hours to days. It is caused by the addition of acidic or basic components. The buffer systems functioning in blood plasma include plasma proteins, phosphate,. What Are The 3 Buffer Systems.
From www.slideserve.com
PPT Fluids and Electrolytes PowerPoint Presentation, free download What Are The 3 Buffer Systems It is caused by the addition of acidic or basic components. Blood ph is regulated by multiple homeostatic mechanisms, including chemical buffers, respiration, and the kidneys. A buffer is a solution that can withstand changes in ph. The bicarbonate buffer system manages acid/base imbalances and effectively manages the release of excess carbon dioxide as a bi. A solution used to. What Are The 3 Buffer Systems.
From www.slideshare.net
Buffer system What Are The 3 Buffer Systems The bicarbonate buffer system can act within seconds to minutes to counteract changes in ph, while the lungs take minutes, and the kidneys take hours to days. A solution used to stabilize the ph. A buffer is a solution that can withstand changes in ph. The bicarbonate buffer system manages acid/base imbalances and effectively manages the release of excess carbon. What Are The 3 Buffer Systems.
From www.slideserve.com
PPT Blood Gases, pH and Buffer system PowerPoint Presentation ID257615 What Are The 3 Buffer Systems The bicarbonate buffer system manages acid/base imbalances and effectively manages the release of excess carbon dioxide as a bi. A solution used to stabilize the ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. It is caused by the addition of acidic or basic components. A buffer is a solution that. What Are The 3 Buffer Systems.
From www.slideserve.com
PPT Acids and Bases and Buffers PowerPoint Presentation, free What Are The 3 Buffer Systems It is caused by the addition of acidic or basic components. The bicarbonate buffer system manages acid/base imbalances and effectively manages the release of excess carbon dioxide as a bi. A solution used to stabilize the ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Blood ph is regulated by multiple. What Are The 3 Buffer Systems.
From www.slideserve.com
PPT Homeostasis PowerPoint Presentation, free download ID1138529 What Are The 3 Buffer Systems The bicarbonate buffer system can act within seconds to minutes to counteract changes in ph, while the lungs take minutes, and the kidneys take hours to days. Blood ph is regulated by multiple homeostatic mechanisms, including chemical buffers, respiration, and the kidneys. It is caused by the addition of acidic or basic components. A buffer is a solution that can. What Are The 3 Buffer Systems.
From www.slideserve.com
PPT Acid and Base Balance PowerPoint Presentation, free download ID What Are The 3 Buffer Systems The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. A buffer is a solution that can withstand changes in ph. The bicarbonate buffer system manages acid/base imbalances and effectively manages the release of excess carbon dioxide as a bi. A solution used to stabilize the ph. Blood ph is regulated by multiple. What Are The 3 Buffer Systems.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID What Are The 3 Buffer Systems A solution used to stabilize the ph. A buffer is a solution that can withstand changes in ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The bicarbonate buffer system manages acid/base imbalances and effectively manages the release of excess carbon dioxide as a bi. Blood ph is regulated by multiple. What Are The 3 Buffer Systems.
From labpedia.net
Acidbase Balance Part 3 Respiratory Acidosis and Respiratory What Are The 3 Buffer Systems The bicarbonate buffer system can act within seconds to minutes to counteract changes in ph, while the lungs take minutes, and the kidneys take hours to days. It is caused by the addition of acidic or basic components. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Blood ph is regulated by. What Are The 3 Buffer Systems.
From www.youtube.com
Chemical Buffers protein buffer, phosphate buffer system and What Are The 3 Buffer Systems Blood ph is regulated by multiple homeostatic mechanisms, including chemical buffers, respiration, and the kidneys. The bicarbonate buffer system can act within seconds to minutes to counteract changes in ph, while the lungs take minutes, and the kidneys take hours to days. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The. What Are The 3 Buffer Systems.
From www.youtube.com
Introduction to Buffer System Regulation of pH Acid Base Balance What Are The 3 Buffer Systems The bicarbonate buffer system manages acid/base imbalances and effectively manages the release of excess carbon dioxide as a bi. A buffer is a solution that can withstand changes in ph. The bicarbonate buffer system can act within seconds to minutes to counteract changes in ph, while the lungs take minutes, and the kidneys take hours to days. It is caused. What Are The 3 Buffer Systems.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent What Are The 3 Buffer Systems A solution used to stabilize the ph. Blood ph is regulated by multiple homeostatic mechanisms, including chemical buffers, respiration, and the kidneys. A buffer is a solution that can withstand changes in ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The bicarbonate buffer system can act within seconds to minutes. What Are The 3 Buffer Systems.
From www.slideserve.com
PPT MLAB 2401 Clinical Chemistry Keri BrophyMartinez PowerPoint What Are The 3 Buffer Systems The bicarbonate buffer system manages acid/base imbalances and effectively manages the release of excess carbon dioxide as a bi. It is caused by the addition of acidic or basic components. The bicarbonate buffer system can act within seconds to minutes to counteract changes in ph, while the lungs take minutes, and the kidneys take hours to days. The buffer systems. What Are The 3 Buffer Systems.
From www.slideserve.com
PPT Renal Physiology PowerPoint Presentation, free download ID5632772 What Are The 3 Buffer Systems A buffer is a solution that can withstand changes in ph. The bicarbonate buffer system can act within seconds to minutes to counteract changes in ph, while the lungs take minutes, and the kidneys take hours to days. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The bicarbonate buffer system manages. What Are The 3 Buffer Systems.
From www.slideserve.com
PPT Fluid, Electrolyte, and AcidBase Balance PowerPoint Presentation What Are The 3 Buffer Systems It is caused by the addition of acidic or basic components. The bicarbonate buffer system manages acid/base imbalances and effectively manages the release of excess carbon dioxide as a bi. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Blood ph is regulated by multiple homeostatic mechanisms, including chemical buffers, respiration, and. What Are The 3 Buffer Systems.
From www.slideserve.com
PPT Physiological system of blood. Functional importance of blood What Are The 3 Buffer Systems The bicarbonate buffer system can act within seconds to minutes to counteract changes in ph, while the lungs take minutes, and the kidneys take hours to days. Blood ph is regulated by multiple homeostatic mechanisms, including chemical buffers, respiration, and the kidneys. A solution used to stabilize the ph. The buffer systems functioning in blood plasma include plasma proteins, phosphate,. What Are The 3 Buffer Systems.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint What Are The 3 Buffer Systems Blood ph is regulated by multiple homeostatic mechanisms, including chemical buffers, respiration, and the kidneys. The bicarbonate buffer system can act within seconds to minutes to counteract changes in ph, while the lungs take minutes, and the kidneys take hours to days. A solution used to stabilize the ph. It is caused by the addition of acidic or basic components.. What Are The 3 Buffer Systems.
From www.pinterest.com
Understanding Buffer Solution Chemistry A Complete Guide What Are The 3 Buffer Systems The bicarbonate buffer system can act within seconds to minutes to counteract changes in ph, while the lungs take minutes, and the kidneys take hours to days. The bicarbonate buffer system manages acid/base imbalances and effectively manages the release of excess carbon dioxide as a bi. A solution used to stabilize the ph. It is caused by the addition of. What Are The 3 Buffer Systems.
From www.slideserve.com
PPT Acid Base Balance PowerPoint Presentation, free download ID2413658 What Are The 3 Buffer Systems The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. A solution used to stabilize the ph. Blood ph is regulated by multiple homeostatic mechanisms, including chemical buffers, respiration, and the kidneys. A buffer is a solution that can withstand changes in ph. It is caused by the addition of acidic or basic. What Are The 3 Buffer Systems.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID What Are The 3 Buffer Systems Blood ph is regulated by multiple homeostatic mechanisms, including chemical buffers, respiration, and the kidneys. It is caused by the addition of acidic or basic components. The bicarbonate buffer system manages acid/base imbalances and effectively manages the release of excess carbon dioxide as a bi. The bicarbonate buffer system can act within seconds to minutes to counteract changes in ph,. What Are The 3 Buffer Systems.