Br2 + Ki Type Of Reaction . The main concept that must be applied to determine the coefficients (amount of each item) is that there must be. Kbr + i2 = br2 + ki is a single displacement (substitution) reaction where two moles of aqueous potassium bromide [kbr] and one mole. You need to be able to recognize single replacement reactions and be able to break a formula apart into proper cations and anions as. The type of chemical reaction represents above is the single displacement reaction whereby reactive element displaces a. There are three main steps for writing the net ionic equation for ki + br2 = kbr + i2 (potassium. The principal types of reactions involving aromatic rings are substitution, addition, and oxidation. Of these, the most common type is electrophilic substitution. In this lesson, you will learn about a type of reaction called single displacement and how the activity series helps you predict the product of a single displacement reaction.
from www.chegg.com
There are three main steps for writing the net ionic equation for ki + br2 = kbr + i2 (potassium. Of these, the most common type is electrophilic substitution. Kbr + i2 = br2 + ki is a single displacement (substitution) reaction where two moles of aqueous potassium bromide [kbr] and one mole. The type of chemical reaction represents above is the single displacement reaction whereby reactive element displaces a. The principal types of reactions involving aromatic rings are substitution, addition, and oxidation. In this lesson, you will learn about a type of reaction called single displacement and how the activity series helps you predict the product of a single displacement reaction. You need to be able to recognize single replacement reactions and be able to break a formula apart into proper cations and anions as. The main concept that must be applied to determine the coefficients (amount of each item) is that there must be.
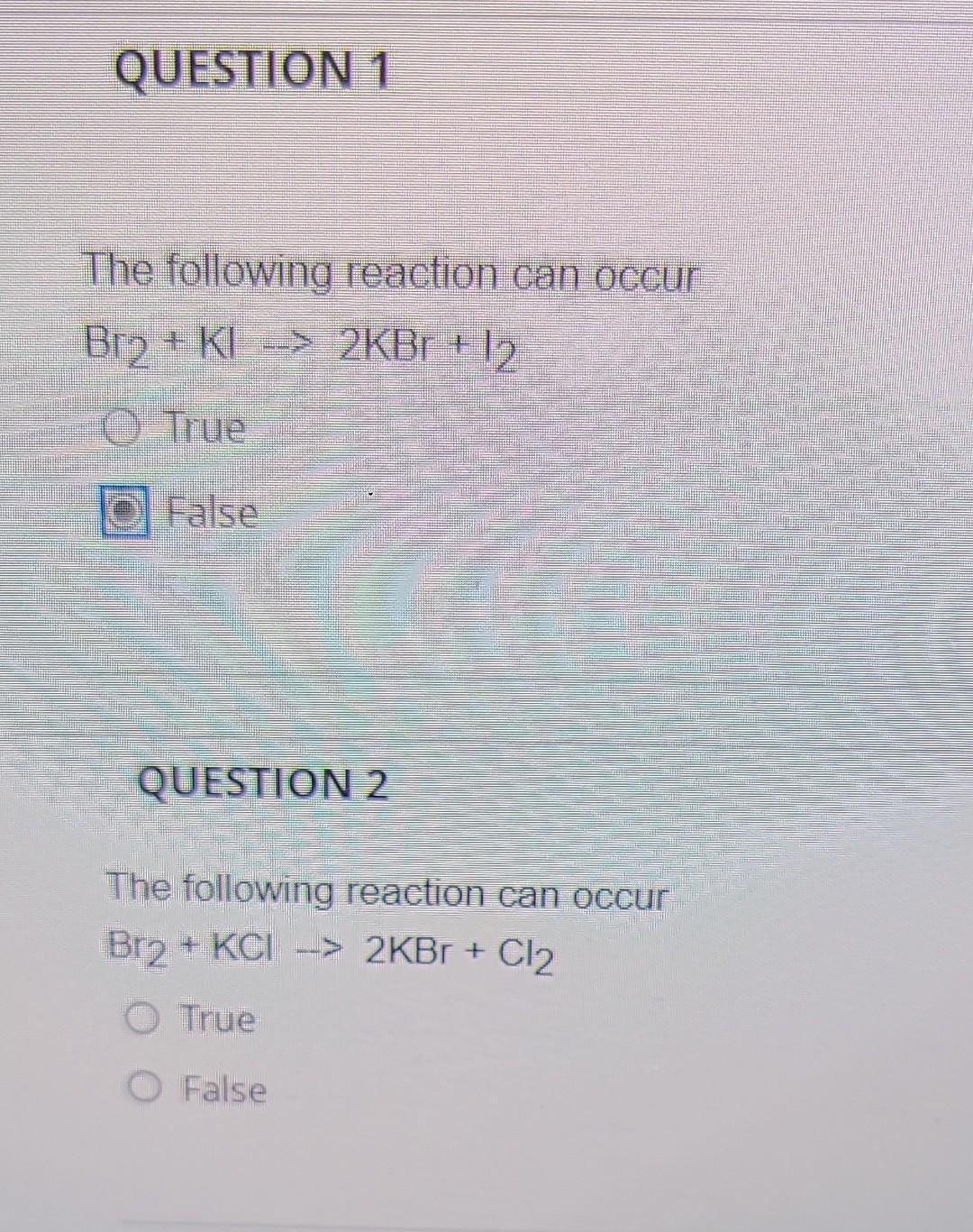
Solved The following reaction can occur Br2+KI→2KBr+12 True
Br2 + Ki Type Of Reaction The principal types of reactions involving aromatic rings are substitution, addition, and oxidation. The main concept that must be applied to determine the coefficients (amount of each item) is that there must be. Of these, the most common type is electrophilic substitution. In this lesson, you will learn about a type of reaction called single displacement and how the activity series helps you predict the product of a single displacement reaction. Kbr + i2 = br2 + ki is a single displacement (substitution) reaction where two moles of aqueous potassium bromide [kbr] and one mole. The type of chemical reaction represents above is the single displacement reaction whereby reactive element displaces a. The principal types of reactions involving aromatic rings are substitution, addition, and oxidation. There are three main steps for writing the net ionic equation for ki + br2 = kbr + i2 (potassium. You need to be able to recognize single replacement reactions and be able to break a formula apart into proper cations and anions as.
From www.youtube.com
Type of Reaction for KI + Br2 = KBr + I2 YouTube Br2 + Ki Type Of Reaction The type of chemical reaction represents above is the single displacement reaction whereby reactive element displaces a. There are three main steps for writing the net ionic equation for ki + br2 = kbr + i2 (potassium. In this lesson, you will learn about a type of reaction called single displacement and how the activity series helps you predict the. Br2 + Ki Type Of Reaction.
From www.youtube.com
Halogen halide demo Cl2, Br2, I2 mixed with I, Br, Cl YouTube Br2 + Ki Type Of Reaction There are three main steps for writing the net ionic equation for ki + br2 = kbr + i2 (potassium. In this lesson, you will learn about a type of reaction called single displacement and how the activity series helps you predict the product of a single displacement reaction. The principal types of reactions involving aromatic rings are substitution, addition,. Br2 + Ki Type Of Reaction.
From www.toppr.com
Identify product obtained by following sequence of reactions 1.HNO3 Br2 + Ki Type Of Reaction You need to be able to recognize single replacement reactions and be able to break a formula apart into proper cations and anions as. There are three main steps for writing the net ionic equation for ki + br2 = kbr + i2 (potassium. The principal types of reactions involving aromatic rings are substitution, addition, and oxidation. The main concept. Br2 + Ki Type Of Reaction.
From www.coursehero.com
[Solved] Predict the major product of this reaction sequence 1. Br2 Br2 + Ki Type Of Reaction Of these, the most common type is electrophilic substitution. You need to be able to recognize single replacement reactions and be able to break a formula apart into proper cations and anions as. The main concept that must be applied to determine the coefficients (amount of each item) is that there must be. The principal types of reactions involving aromatic. Br2 + Ki Type Of Reaction.
From www.transtutors.com
(Get Answer) Transcribed image text 9 A reaction between Br2 and KI Br2 + Ki Type Of Reaction The type of chemical reaction represents above is the single displacement reaction whereby reactive element displaces a. There are three main steps for writing the net ionic equation for ki + br2 = kbr + i2 (potassium. You need to be able to recognize single replacement reactions and be able to break a formula apart into proper cations and anions. Br2 + Ki Type Of Reaction.
From www.ochemtools.com
Organic Chemistry! app Br2 Br2 + Ki Type Of Reaction You need to be able to recognize single replacement reactions and be able to break a formula apart into proper cations and anions as. The type of chemical reaction represents above is the single displacement reaction whereby reactive element displaces a. In this lesson, you will learn about a type of reaction called single displacement and how the activity series. Br2 + Ki Type Of Reaction.
From www.numerade.com
SOLVED Give the major product of the following reaction Br2 Give the Br2 + Ki Type Of Reaction In this lesson, you will learn about a type of reaction called single displacement and how the activity series helps you predict the product of a single displacement reaction. The type of chemical reaction represents above is the single displacement reaction whereby reactive element displaces a. Of these, the most common type is electrophilic substitution. Kbr + i2 = br2. Br2 + Ki Type Of Reaction.
From www.coursehero.com
[Solved] Predict the major product of this reaction 1 equiv. Br2, hv Br2 + Ki Type Of Reaction You need to be able to recognize single replacement reactions and be able to break a formula apart into proper cations and anions as. The principal types of reactions involving aromatic rings are substitution, addition, and oxidation. Kbr + i2 = br2 + ki is a single displacement (substitution) reaction where two moles of aqueous potassium bromide [kbr] and one. Br2 + Ki Type Of Reaction.
From www.numerade.com
Classify each chemical reaction Reaction Type of reaction (check all Br2 + Ki Type Of Reaction The principal types of reactions involving aromatic rings are substitution, addition, and oxidation. Of these, the most common type is electrophilic substitution. There are three main steps for writing the net ionic equation for ki + br2 = kbr + i2 (potassium. You need to be able to recognize single replacement reactions and be able to break a formula apart. Br2 + Ki Type Of Reaction.
From www.chegg.com
Solved Given the following reactions NaCl (aq) + Br2 (1) Br2 + Ki Type Of Reaction There are three main steps for writing the net ionic equation for ki + br2 = kbr + i2 (potassium. The principal types of reactions involving aromatic rings are substitution, addition, and oxidation. Kbr + i2 = br2 + ki is a single displacement (substitution) reaction where two moles of aqueous potassium bromide [kbr] and one mole. The type of. Br2 + Ki Type Of Reaction.
From www.youtube.com
How to Balance KI + Br2 = KBr + I2 (Potassium iodide + Bromine gas Br2 + Ki Type Of Reaction The type of chemical reaction represents above is the single displacement reaction whereby reactive element displaces a. The principal types of reactions involving aromatic rings are substitution, addition, and oxidation. There are three main steps for writing the net ionic equation for ki + br2 = kbr + i2 (potassium. Kbr + i2 = br2 + ki is a single. Br2 + Ki Type Of Reaction.
From www.numerade.com
SOLVED Give the major product of the following reactions with the Br2 + Ki Type Of Reaction The type of chemical reaction represents above is the single displacement reaction whereby reactive element displaces a. There are three main steps for writing the net ionic equation for ki + br2 = kbr + i2 (potassium. Of these, the most common type is electrophilic substitution. The principal types of reactions involving aromatic rings are substitution, addition, and oxidation. The. Br2 + Ki Type Of Reaction.
From www.chegg.com
Solved What is the product of the following reaction? Br2 Br2 + Ki Type Of Reaction In this lesson, you will learn about a type of reaction called single displacement and how the activity series helps you predict the product of a single displacement reaction. There are three main steps for writing the net ionic equation for ki + br2 = kbr + i2 (potassium. Of these, the most common type is electrophilic substitution. The type. Br2 + Ki Type Of Reaction.
From www.toppr.com
What is the major organic product of the following reaction? Ethyl Br2 + Ki Type Of Reaction You need to be able to recognize single replacement reactions and be able to break a formula apart into proper cations and anions as. The main concept that must be applied to determine the coefficients (amount of each item) is that there must be. The type of chemical reaction represents above is the single displacement reaction whereby reactive element displaces. Br2 + Ki Type Of Reaction.
From www.coursehero.com
[Solved] The reaction of Br2 with 1methylcyclohexene, in the presence Br2 + Ki Type Of Reaction There are three main steps for writing the net ionic equation for ki + br2 = kbr + i2 (potassium. The main concept that must be applied to determine the coefficients (amount of each item) is that there must be. You need to be able to recognize single replacement reactions and be able to break a formula apart into proper. Br2 + Ki Type Of Reaction.
From www.youtube.com
Electrophilic Addition Mechanism with Bromine YouTube Br2 + Ki Type Of Reaction The main concept that must be applied to determine the coefficients (amount of each item) is that there must be. Of these, the most common type is electrophilic substitution. The type of chemical reaction represents above is the single displacement reaction whereby reactive element displaces a. You need to be able to recognize single replacement reactions and be able to. Br2 + Ki Type Of Reaction.
From www.youtube.com
How to Write the Net Ionic Equation for KI + Cl2 = KCl + I2 YouTube Br2 + Ki Type Of Reaction You need to be able to recognize single replacement reactions and be able to break a formula apart into proper cations and anions as. There are three main steps for writing the net ionic equation for ki + br2 = kbr + i2 (potassium. Of these, the most common type is electrophilic substitution. The principal types of reactions involving aromatic. Br2 + Ki Type Of Reaction.
From www.youtube.com
How to Write the Net Ionic Equation for KI + Br2 = KBr + I2 YouTube Br2 + Ki Type Of Reaction The type of chemical reaction represents above is the single displacement reaction whereby reactive element displaces a. In this lesson, you will learn about a type of reaction called single displacement and how the activity series helps you predict the product of a single displacement reaction. The principal types of reactions involving aromatic rings are substitution, addition, and oxidation. Kbr. Br2 + Ki Type Of Reaction.
From www.chegg.com
Solved Br2 What is a major product of this reaction? FeBr3 Br2 + Ki Type Of Reaction The principal types of reactions involving aromatic rings are substitution, addition, and oxidation. Kbr + i2 = br2 + ki is a single displacement (substitution) reaction where two moles of aqueous potassium bromide [kbr] and one mole. Of these, the most common type is electrophilic substitution. The type of chemical reaction represents above is the single displacement reaction whereby reactive. Br2 + Ki Type Of Reaction.
From www.chegg.com
Solved The following reaction can occur Br2+KI→2KBr+l2 True Br2 + Ki Type Of Reaction The principal types of reactions involving aromatic rings are substitution, addition, and oxidation. Kbr + i2 = br2 + ki is a single displacement (substitution) reaction where two moles of aqueous potassium bromide [kbr] and one mole. You need to be able to recognize single replacement reactions and be able to break a formula apart into proper cations and anions. Br2 + Ki Type Of Reaction.
From www.bartleby.com
Answered Write the NetIonic Reaction for the… bartleby Br2 + Ki Type Of Reaction The principal types of reactions involving aromatic rings are substitution, addition, and oxidation. Of these, the most common type is electrophilic substitution. Kbr + i2 = br2 + ki is a single displacement (substitution) reaction where two moles of aqueous potassium bromide [kbr] and one mole. The main concept that must be applied to determine the coefficients (amount of each. Br2 + Ki Type Of Reaction.
From www.chegg.com
Solved The following reaction can occur Br2+KI→2KBr+l2 True Br2 + Ki Type Of Reaction Kbr + i2 = br2 + ki is a single displacement (substitution) reaction where two moles of aqueous potassium bromide [kbr] and one mole. In this lesson, you will learn about a type of reaction called single displacement and how the activity series helps you predict the product of a single displacement reaction. The main concept that must be applied. Br2 + Ki Type Of Reaction.
From www.chegg.com
Solved The following reaction can occur Br2+KI→2KBr+12 True Br2 + Ki Type Of Reaction In this lesson, you will learn about a type of reaction called single displacement and how the activity series helps you predict the product of a single displacement reaction. Of these, the most common type is electrophilic substitution. You need to be able to recognize single replacement reactions and be able to break a formula apart into proper cations and. Br2 + Ki Type Of Reaction.
From www.youtube.com
Half Reaction Method, Balancing Redox Reactions In Basic & Acidic Br2 + Ki Type Of Reaction Of these, the most common type is electrophilic substitution. The principal types of reactions involving aromatic rings are substitution, addition, and oxidation. The type of chemical reaction represents above is the single displacement reaction whereby reactive element displaces a. In this lesson, you will learn about a type of reaction called single displacement and how the activity series helps you. Br2 + Ki Type Of Reaction.
From mungfali.com
Alkene Br2 H2O Br2 + Ki Type Of Reaction The principal types of reactions involving aromatic rings are substitution, addition, and oxidation. In this lesson, you will learn about a type of reaction called single displacement and how the activity series helps you predict the product of a single displacement reaction. The type of chemical reaction represents above is the single displacement reaction whereby reactive element displaces a. Kbr. Br2 + Ki Type Of Reaction.
From www.youtube.com
Br2=BrO3^+Br^ balance the redox reaction in a basic medium. br2=bro3 Br2 + Ki Type Of Reaction The type of chemical reaction represents above is the single displacement reaction whereby reactive element displaces a. Kbr + i2 = br2 + ki is a single displacement (substitution) reaction where two moles of aqueous potassium bromide [kbr] and one mole. Of these, the most common type is electrophilic substitution. There are three main steps for writing the net ionic. Br2 + Ki Type Of Reaction.
From www.chegg.com
Solved If one equivalent of Br2 reacts with the given Br2 + Ki Type Of Reaction The principal types of reactions involving aromatic rings are substitution, addition, and oxidation. In this lesson, you will learn about a type of reaction called single displacement and how the activity series helps you predict the product of a single displacement reaction. The type of chemical reaction represents above is the single displacement reaction whereby reactive element displaces a. You. Br2 + Ki Type Of Reaction.
From byjus.com
8. Benzene + ( HNO2 / conc. H2SO4) A + Br2/Fe (dark) B + Sn/HCl + NaNO2 Br2 + Ki Type Of Reaction Kbr + i2 = br2 + ki is a single displacement (substitution) reaction where two moles of aqueous potassium bromide [kbr] and one mole. The type of chemical reaction represents above is the single displacement reaction whereby reactive element displaces a. The main concept that must be applied to determine the coefficients (amount of each item) is that there must. Br2 + Ki Type Of Reaction.
From www.coursehero.com
[Solved] Br2/hv What is the major product of the following reaction? Br Br2 + Ki Type Of Reaction The principal types of reactions involving aromatic rings are substitution, addition, and oxidation. The main concept that must be applied to determine the coefficients (amount of each item) is that there must be. You need to be able to recognize single replacement reactions and be able to break a formula apart into proper cations and anions as. Kbr + i2. Br2 + Ki Type Of Reaction.
From www.numerade.com
SOLVED Br2, NaOH Acetone NaOH 1(1CyclopentenIyl)1ethanone Br2 + Ki Type Of Reaction The main concept that must be applied to determine the coefficients (amount of each item) is that there must be. You need to be able to recognize single replacement reactions and be able to break a formula apart into proper cations and anions as. Kbr + i2 = br2 + ki is a single displacement (substitution) reaction where two moles. Br2 + Ki Type Of Reaction.
From www.youtube.com
Redox balance Br2 = BrO3 + Br Ion electron method Half reaction Br2 + Ki Type Of Reaction The principal types of reactions involving aromatic rings are substitution, addition, and oxidation. The main concept that must be applied to determine the coefficients (amount of each item) is that there must be. The type of chemical reaction represents above is the single displacement reaction whereby reactive element displaces a. Kbr + i2 = br2 + ki is a single. Br2 + Ki Type Of Reaction.
From www.masterorganicchemistry.com
Bromination of alkenes with Br2 to give dibromides Master Organic Br2 + Ki Type Of Reaction You need to be able to recognize single replacement reactions and be able to break a formula apart into proper cations and anions as. The main concept that must be applied to determine the coefficients (amount of each item) is that there must be. The type of chemical reaction represents above is the single displacement reaction whereby reactive element displaces. Br2 + Ki Type Of Reaction.
From byjus.com
Does phenol react with bromine? Br2 + Ki Type Of Reaction You need to be able to recognize single replacement reactions and be able to break a formula apart into proper cations and anions as. In this lesson, you will learn about a type of reaction called single displacement and how the activity series helps you predict the product of a single displacement reaction. Of these, the most common type is. Br2 + Ki Type Of Reaction.
From questions-in.kunduz.com
Br . Br2 KI CCIA Br ii) HBO CCHA ILI Corre... Organic Chemistry Br2 + Ki Type Of Reaction The principal types of reactions involving aromatic rings are substitution, addition, and oxidation. You need to be able to recognize single replacement reactions and be able to break a formula apart into proper cations and anions as. Kbr + i2 = br2 + ki is a single displacement (substitution) reaction where two moles of aqueous potassium bromide [kbr] and one. Br2 + Ki Type Of Reaction.
From www.numerade.com
SOLVED Complete the following single displacement reactions using the Br2 + Ki Type Of Reaction You need to be able to recognize single replacement reactions and be able to break a formula apart into proper cations and anions as. Of these, the most common type is electrophilic substitution. The main concept that must be applied to determine the coefficients (amount of each item) is that there must be. The principal types of reactions involving aromatic. Br2 + Ki Type Of Reaction.