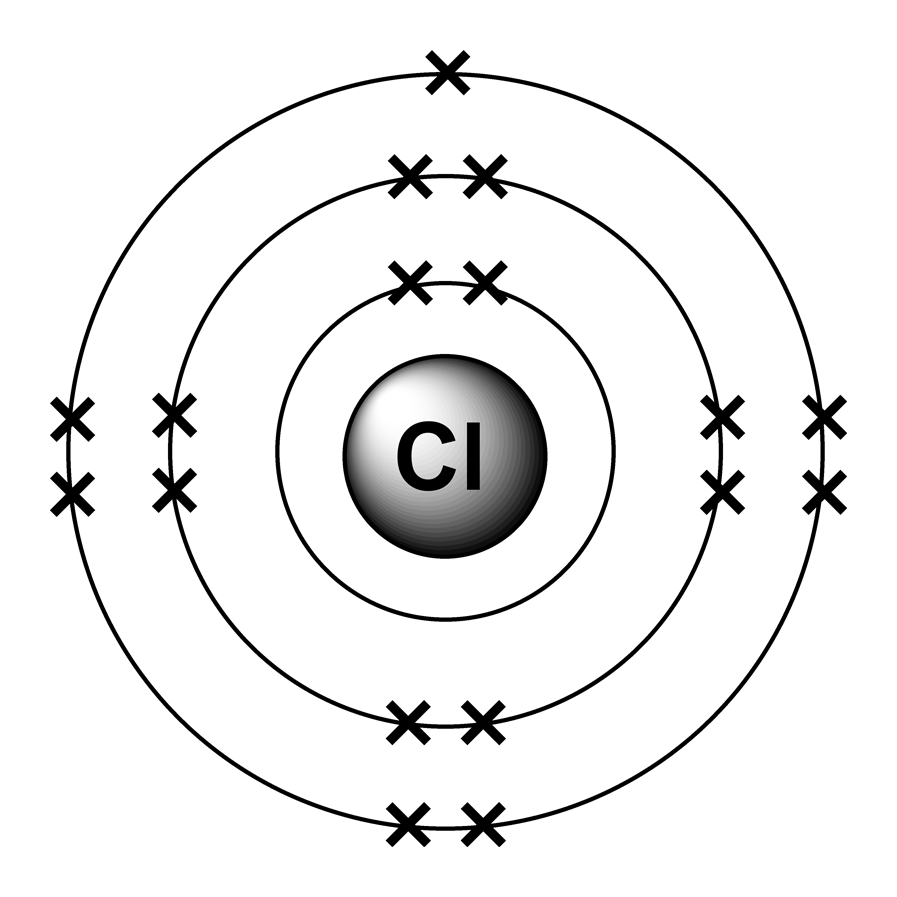
Chlorine Electron Arrangement . Counting valence electrons in chlorine atoms from the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons does a The first two electrons will go in the \(n=1\) level. To represent the lewis dot diagram for chlorine, the. This diagram is a visual representation of the bonding and electron distribution in a chlorine molecule. Chlorine has an atomic number of 17, meaning it has 17 protons and 17 electrons. Solution chlorine has 17 electrons. What is the electron arrangement of chlorine? Chlorine has an atomic number of 17, which means it has 17 protons and. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Electron configuration of chlorine is [ne] 3s2 3p5. Full ground state electron configuration: Determine the number of valence electrons in a chlorine atom. It is an extremely reactive element and a strong oxidising agent: Two is the maximum number of electrons for the level so the.
from www.benjamin-mills.com
It is an extremely reactive element and a strong oxidising agent: Chlorine has an atomic number of 17, meaning it has 17 protons and 17 electrons. Full ground state electron configuration: To represent the lewis dot diagram for chlorine, the. Electron configuration of chlorine is [ne] 3s2 3p5. What is the electron arrangement of chlorine? Solution chlorine has 17 electrons. The first two electrons will go in the \(n=1\) level. Two is the maximum number of electrons for the level so the. Determine the number of valence electrons in a chlorine atom.
Electron configurations
Chlorine Electron Arrangement Solution chlorine has 17 electrons. Full ground state electron configuration: Determine the number of valence electrons in a chlorine atom. Electron configuration of chlorine is [ne] 3s2 3p5. The first two electrons will go in the \(n=1\) level. Chlorine has an atomic number of 17, meaning it has 17 protons and 17 electrons. Counting valence electrons in chlorine atoms from the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons does a Solution chlorine has 17 electrons. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). What is the electron arrangement of chlorine? Chlorine has an atomic number of 17, which means it has 17 protons and. Two is the maximum number of electrons for the level so the. This diagram is a visual representation of the bonding and electron distribution in a chlorine molecule. It is an extremely reactive element and a strong oxidising agent: To represent the lewis dot diagram for chlorine, the.
From www.shutterstock.com
3.722 Atom structure of chlorine Görseli, Stok Fotoğraflar ve Vektörler Chlorine Electron Arrangement The first two electrons will go in the \(n=1\) level. Two is the maximum number of electrons for the level so the. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Full ground state electron configuration: Counting valence electrons in chlorine atoms from the. Chlorine Electron Arrangement.
From favpng.com
Atom Bohr Model Electron Configuration Chlorine, PNG, 1000x1000px, Atom Chlorine Electron Arrangement Determine the number of valence electrons in a chlorine atom. Chlorine has an atomic number of 17, meaning it has 17 protons and 17 electrons. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Two is the maximum number of electrons for the level. Chlorine Electron Arrangement.
From www.alamy.com
Chlorine (Cl). Diagram of the nuclear composition and electron Chlorine Electron Arrangement Counting valence electrons in chlorine atoms from the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons does a The first two electrons will go in the \(n=1\) level. Two is the maximum number of electrons for the level so the. To represent the lewis dot diagram for chlorine, the. Chlorine has. Chlorine Electron Arrangement.
From egpat.com
Lewis dot structure How to write? Chlorine Electron Arrangement Two is the maximum number of electrons for the level so the. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Chlorine has an atomic number of 17, which means it has 17 protons and. Electron configuration of chlorine is [ne] 3s2 3p5. What. Chlorine Electron Arrangement.
From favpng.com
Electron Configuration Atomic Orbital Chlorine Chemistry, PNG Chlorine Electron Arrangement In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). To represent the lewis dot diagram for chlorine, the. The first two electrons will go in the \(n=1\) level. It is an extremely reactive element and a strong oxidising agent: Electron configuration of chlorine is. Chlorine Electron Arrangement.
From womackthille.blogspot.com
Expanded Electron Configuration of Chlorine Womack Thille Chlorine Electron Arrangement Chlorine has an atomic number of 17, meaning it has 17 protons and 17 electrons. Two is the maximum number of electrons for the level so the. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Electron configuration of chlorine is [ne] 3s2 3p5.. Chlorine Electron Arrangement.
From material-properties.org
Chlorine Protons Neutrons Electrons Electron Configuration Chlorine Electron Arrangement Determine the number of valence electrons in a chlorine atom. Counting valence electrons in chlorine atoms from the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons does a What is the electron arrangement of chlorine? Electron configuration of chlorine is [ne] 3s2 3p5. Chlorine has an atomic number of 17, meaning. Chlorine Electron Arrangement.
From www.alamy.com
Molecular Model of Chlorine (Cl2) Molecule. Vector Illustration Stock Chlorine Electron Arrangement Counting valence electrons in chlorine atoms from the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons does a To represent the lewis dot diagram for chlorine, the. This diagram is a visual representation of the bonding and electron distribution in a chlorine molecule. Full ground state electron configuration: Two is the. Chlorine Electron Arrangement.
From sciencenotes.org
Chlorine Facts Chlorine Electron Arrangement Solution chlorine has 17 electrons. Electron configuration of chlorine is [ne] 3s2 3p5. Two is the maximum number of electrons for the level so the. This diagram is a visual representation of the bonding and electron distribution in a chlorine molecule. Counting valence electrons in chlorine atoms from the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence. Chlorine Electron Arrangement.
From www.alamy.com
Chlorine (Cl). Diagram of the nuclear composition and electron Chlorine Electron Arrangement What is the electron arrangement of chlorine? Counting valence electrons in chlorine atoms from the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons does a It is an extremely reactive element and a strong oxidising agent: In order to write the chlorine electron configuration we first need to know the number. Chlorine Electron Arrangement.
From www.britannica.com
Halogen Elements, Examples, Properties, Uses, & Facts Britannica Chlorine Electron Arrangement Chlorine has an atomic number of 17, which means it has 17 protons and. Counting valence electrons in chlorine atoms from the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons does a Determine the number of valence electrons in a chlorine atom. Two is the maximum number of electrons for the. Chlorine Electron Arrangement.
From flatdisk24.pythonanywhere.com
How To Build A Chlorine Atom Flatdisk24 Chlorine Electron Arrangement The first two electrons will go in the \(n=1\) level. Solution chlorine has 17 electrons. What is the electron arrangement of chlorine? To represent the lewis dot diagram for chlorine, the. This diagram is a visual representation of the bonding and electron distribution in a chlorine molecule. It is an extremely reactive element and a strong oxidising agent: Full ground. Chlorine Electron Arrangement.
From www.dreamstime.com
Electron Arrangement Chlorine Stock Illustrations 1 Electron Chlorine Electron Arrangement This diagram is a visual representation of the bonding and electron distribution in a chlorine molecule. Solution chlorine has 17 electrons. Two is the maximum number of electrons for the level so the. To represent the lewis dot diagram for chlorine, the. The first two electrons will go in the \(n=1\) level. Counting valence electrons in chlorine atoms from the. Chlorine Electron Arrangement.
From www.shutterstock.com
Atom Chlorine This Diagram Shows Electron Stock Vector 328668782 Chlorine Electron Arrangement Two is the maximum number of electrons for the level so the. Solution chlorine has 17 electrons. Determine the number of valence electrons in a chlorine atom. What is the electron arrangement of chlorine? Electron configuration of chlorine is [ne] 3s2 3p5. The first two electrons will go in the \(n=1\) level. Chlorine has an atomic number of 17, which. Chlorine Electron Arrangement.
From commons.wikimedia.org
FileElectron shell 017 chlorine.png Wikimedia Commons Chlorine Electron Arrangement To represent the lewis dot diagram for chlorine, the. Chlorine has an atomic number of 17, which means it has 17 protons and. What is the electron arrangement of chlorine? This diagram is a visual representation of the bonding and electron distribution in a chlorine molecule. Solution chlorine has 17 electrons. It is an extremely reactive element and a strong. Chlorine Electron Arrangement.
From www.sciencephoto.com
Chlorine, atomic structure Stock Image C018/3698 Science Photo Library Chlorine Electron Arrangement The first two electrons will go in the \(n=1\) level. Full ground state electron configuration: Electron configuration of chlorine is [ne] 3s2 3p5. To represent the lewis dot diagram for chlorine, the. Solution chlorine has 17 electrons. This diagram is a visual representation of the bonding and electron distribution in a chlorine molecule. What is the electron arrangement of chlorine?. Chlorine Electron Arrangement.
From chemtech-us.com
15 Interesting Facts About Chlorine Chlorine Electron Arrangement Determine the number of valence electrons in a chlorine atom. It is an extremely reactive element and a strong oxidising agent: To represent the lewis dot diagram for chlorine, the. Counting valence electrons in chlorine atoms from the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons does a Full ground state. Chlorine Electron Arrangement.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Chlorine Electron Arrangement Determine the number of valence electrons in a chlorine atom. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). What is the electron arrangement of chlorine? Solution chlorine has 17 electrons. The first two electrons will go in the \(n=1\) level. Counting valence electrons. Chlorine Electron Arrangement.
From utedzz.blogspot.com
Periodic Table Chlorine Atomic Number Periodic Table Timeline Chlorine Electron Arrangement Two is the maximum number of electrons for the level so the. Chlorine has an atomic number of 17, which means it has 17 protons and. The first two electrons will go in the \(n=1\) level. It is an extremely reactive element and a strong oxidising agent: In order to write the chlorine electron configuration we first need to know. Chlorine Electron Arrangement.
From brokeasshome.com
Periodic Table Chlorine Electrons Chlorine Electron Arrangement Two is the maximum number of electrons for the level so the. What is the electron arrangement of chlorine? In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). This diagram is a visual representation of the bonding and electron distribution in a chlorine molecule.. Chlorine Electron Arrangement.
From slideplayer.com
Ionic Bonding. ppt download Chlorine Electron Arrangement This diagram is a visual representation of the bonding and electron distribution in a chlorine molecule. Determine the number of valence electrons in a chlorine atom. The first two electrons will go in the \(n=1\) level. To represent the lewis dot diagram for chlorine, the. What is the electron arrangement of chlorine? Solution chlorine has 17 electrons. Electron configuration of. Chlorine Electron Arrangement.
From basichemistry.blogspot.com
Basic Chemistry October 2012 Chlorine Electron Arrangement This diagram is a visual representation of the bonding and electron distribution in a chlorine molecule. What is the electron arrangement of chlorine? Electron configuration of chlorine is [ne] 3s2 3p5. Chlorine has an atomic number of 17, meaning it has 17 protons and 17 electrons. Full ground state electron configuration: To represent the lewis dot diagram for chlorine, the.. Chlorine Electron Arrangement.
From brainly.in
Draw the atomic structure of a chlorine ion Brainly.in Chlorine Electron Arrangement In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). It is an extremely reactive element and a strong oxidising agent: Full ground state electron configuration: Solution chlorine has 17 electrons. This diagram is a visual representation of the bonding and electron distribution in a. Chlorine Electron Arrangement.
From www.benjamin-mills.com
Electron configurations Chlorine Electron Arrangement Counting valence electrons in chlorine atoms from the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons does a In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Full ground state electron configuration: Chlorine has an. Chlorine Electron Arrangement.
From periodictable.me
How To Find The Electron Configuration For Chlorine Dynamic Periodic Chlorine Electron Arrangement The first two electrons will go in the \(n=1\) level. To represent the lewis dot diagram for chlorine, the. It is an extremely reactive element and a strong oxidising agent: Solution chlorine has 17 electrons. Electron configuration of chlorine is [ne] 3s2 3p5. Full ground state electron configuration: In order to write the chlorine electron configuration we first need to. Chlorine Electron Arrangement.
From www.thoughtco.com
Atoms Diagrams Electron Configurations of Elements Chlorine Electron Arrangement Two is the maximum number of electrons for the level so the. This diagram is a visual representation of the bonding and electron distribution in a chlorine molecule. To represent the lewis dot diagram for chlorine, the. Chlorine has an atomic number of 17, meaning it has 17 protons and 17 electrons. Determine the number of valence electrons in a. Chlorine Electron Arrangement.
From www.sciencephoto.com
Chlorine electron configuration Stock Image C029/5025 Science Chlorine Electron Arrangement Determine the number of valence electrons in a chlorine atom. To represent the lewis dot diagram for chlorine, the. Solution chlorine has 17 electrons. Electron configuration of chlorine is [ne] 3s2 3p5. Chlorine has an atomic number of 17, which means it has 17 protons and. Two is the maximum number of electrons for the level so the. In order. Chlorine Electron Arrangement.
From www.dreamstime.com
Diagram Representation Element Chlorine Stock Illustrations 1 Diagram Chlorine Electron Arrangement This diagram is a visual representation of the bonding and electron distribution in a chlorine molecule. To represent the lewis dot diagram for chlorine, the. Electron configuration of chlorine is [ne] 3s2 3p5. Chlorine has an atomic number of 17, meaning it has 17 protons and 17 electrons. What is the electron arrangement of chlorine? Determine the number of valence. Chlorine Electron Arrangement.
From www.youtube.com
Chlorine Electron Configuration YouTube Chlorine Electron Arrangement Chlorine has an atomic number of 17, meaning it has 17 protons and 17 electrons. Determine the number of valence electrons in a chlorine atom. What is the electron arrangement of chlorine? Electron configuration of chlorine is [ne] 3s2 3p5. Two is the maximum number of electrons for the level so the. It is an extremely reactive element and a. Chlorine Electron Arrangement.
From carmanchewchem.blogspot.com
Chemistry Atomic Structure Valency Chlorine Electron Arrangement This diagram is a visual representation of the bonding and electron distribution in a chlorine molecule. Chlorine has an atomic number of 17, meaning it has 17 protons and 17 electrons. Electron configuration of chlorine is [ne] 3s2 3p5. Solution chlorine has 17 electrons. To represent the lewis dot diagram for chlorine, the. Counting valence electrons in chlorine atoms from. Chlorine Electron Arrangement.
From www.expii.com
Ions — Definition & Overview Expii Chlorine Electron Arrangement Electron configuration of chlorine is [ne] 3s2 3p5. Chlorine has an atomic number of 17, which means it has 17 protons and. Determine the number of valence electrons in a chlorine atom. Solution chlorine has 17 electrons. This diagram is a visual representation of the bonding and electron distribution in a chlorine molecule. What is the electron arrangement of chlorine?. Chlorine Electron Arrangement.
From orderdewaltdw718.blogspot.com
45 atomic orbital diagram for chlorine Diagram Online Chlorine Electron Arrangement What is the electron arrangement of chlorine? In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Solution chlorine has 17 electrons. The first two electrons will go in the \(n=1\) level. Electron configuration of chlorine is [ne] 3s2 3p5. Two is the maximum number. Chlorine Electron Arrangement.
From www.breakingatom.com
Chlorine (Cl) Atomic Number 17 Chlorine Electron Arrangement Full ground state electron configuration: Counting valence electrons in chlorine atoms from the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons does a Electron configuration of chlorine is [ne] 3s2 3p5. This diagram is a visual representation of the bonding and electron distribution in a chlorine molecule. To represent the lewis. Chlorine Electron Arrangement.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image Chlorine Electron Arrangement Electron configuration of chlorine is [ne] 3s2 3p5. Chlorine has an atomic number of 17, which means it has 17 protons and. Determine the number of valence electrons in a chlorine atom. To represent the lewis dot diagram for chlorine, the. In order to write the chlorine electron configuration we first need to know the number of electrons for the. Chlorine Electron Arrangement.
From valenceelectrons.com
How Many Valence Electrons Does Chlorine (Cl) Have? Chlorine Electron Arrangement It is an extremely reactive element and a strong oxidising agent: The first two electrons will go in the \(n=1\) level. Counting valence electrons in chlorine atoms from the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons does a Two is the maximum number of electrons for the level so the.. Chlorine Electron Arrangement.